Off-Site Modular Fabrication Of Biopharma Facilities — What You Need To Know
By Hector M. Samper, principal and strategic advisor, Global Strategic Sourcing Solutions (GSSS)

The next frontier in construction delivery method is here, and it will continue to evolve as the biopharma industry demands innovative solutions to optimize drug development costs. The Modular Facilities in Pharmaceutical/Biotechnology Industry, 2017-2030 report notes that the biopharma modular facilities market is anticipated to grow at an annualized rate of 8.9 percent between 2020 and 2030.1 This projection is being driven by current market conditions that challenge traditional building methods and open opportunities to improve construction delivery practices. Off-site construction and modularization are demonstrating tremendous potential to provide the industry with the agility it needs to speed time to market.
However, pharma and biotech companies need to thoroughly understand the modular process to evaluate its merits and develop an early execution plan. While there are fundamental measures, such as a controlled environment that guarantees cost, schedule, and quality, that tilt the balance toward modularization, all elements of the project execution plan must be analyzed to reach an informed decision.
Potential Benefits Of Off-site Modular Fabrication:
Modular process design and fabrication is a very specific approach to building process systems and plants, which involves designing the systems into portable skids. These skids are self-contained units that can be stacked or rearranged in different formations to add to existing plants or form entire manufacturing plants.2 The following are examples of some major advantages of modular systems:
- Increased productivity: Tools and machinery are readily available and within reach.
- Controlled environment: Sizes, shapes, and finish quality are guaranteed.
- Efficient assembly: Products arrive nearly assembled, requiring limited installation.
- Sustainable and optimum workforce: Use of construction trades is reduced, and the fabrication labor force develops focused skills and expertise.
- Elimination of supply contingency: Materials cut to precise sizes eliminates waste.
- Standardized but customized: Early planning allows for customization, while modularization will support standardization. Building codes and standards are aligned with existing surrounding facilities, unless it is a greenfield site.
- Minimum manufacturing disruption: More flexibility is available in situations where production downtime is not possible.
- Reduction of manufacturing area: Closed modular systems design can reduce required manufacturing area and utility requirements.
- Energy efficiency: Modular facilities are said to be associated with reduction in energy consumption.3
- Schedule savings can be as high a 20 percent.4
Identifying Risks And Opportunities
While transferring unpredictable on-site risks to controlled offsite facilities is an inherent benefit of modularization, the pros and cons of modular building construction need to be weighed against more traditional methods to achieve the project goals. The following are crucial factors that need to be considered carefully.
- Project Logistics: Understanding and planning the transportation route and on-site logistics are critical to ensure that the modules conform to shipping requirements through authorized and approved routes, whether it is by ocean, land, and/or rail freight.
- Virtual Project Team and Resources: It is important to recognize communication barriers due to time zones, culture, language, workflow, and even tools and systems. It is crucial to recognize these challenges to establish clear lines of communication and develop a mitigation plan.
- Defining the Value Proposition: Not having a preconceived notion is essential to performing a non-biased evaluation. Commonly used project design and construction execution strategies must be closely evaluated and compared to the potential benefits of modularization to identify opportunities and define the value-add.
- Material Controls: The client and the modular fabricator need to closely coordinate the materials and equipment flow and just-in-time delivery to align with the fabrication shop. This is key to developing an early equipment delivery schedule.
- Overall Project Schedule Scope: Care must be taken to ensure enough overall schedule time is allotted for proper planning and coordination. Further, the schedule must be maintained and updated on a regular basis.
- Timely Design Coordination: This will always be a challenge, critical to defining the actions and interactions of individuals and groups to monitor design progress as a whole, especially defining the evolution of the design phases from concept to basic and detailed design and approved for construction.
- Fabrication and Delivery Sequence: This is crucial to ensure on-site installation flows smoothly with field activities. Plan for module interconnection issues and the right resources to support and answer to field conditions.
- Factory Acceptance Test (FAT) and Site Acceptance Test (SAT) Alignment: It is essential to define with the equipment vendor the acceptance test criteria at the three parties’ locations: equipment vendor shop, modular fabricator shop, and the owner’s site. This adds a third dimension to the traditional FAT and SAT activities.
It is critical to take a holistic approach to analyzing the benefits and risks of applying modularization and to clearly define and communicate the value proposition for such an approach.
Biopharma Trends Impacting The Growth Of Off-site Modular Fabrication
Industry 4.0 and emerging technologies, coupled with increasing pressure to improve efficiency, decrease operating costs, and speed up the drug development process, are key reasons to consider and evaluate modular solutions. Measurable benefits of off-site construction are becoming more evident and it is increasingly being seen as a viable method for delivering a greater degree of predictability. The following are some examples3 of biopharma industry trends that are propelling the use of off-site module fabrication:
- As some major biological drugs are fast approaching their patent cliffs, the increasing demand for biosimilars will spur manufacturing models that are smaller, more efficient, and more flexible.
- Factories within a pod, as demonstrated by portable, continuous, miniature and modular systems (PCCMs), help facilitate drug production and distribution on demand (e.g., to support personalized medicine).
- Monoclonal antibodies (mAbs) are the most successful class of biopharmaceuticals today, as sales are expected to cross $125 billion by 2020. Changes in the production process, such as greater reliance on single-use technology (SUT), combined with modular construction, can enable rapid assembly of production facilities5 to facilitate speed to market.
- Biopharma CMOs are anticipated to expand capacity, as their growth in revenue is tracking that of the biopharmaceutical sector, at more than 12 percent annually.6
- Emerging trends capitalizing on the use of modular facilities include single-use systems used in continuous production campaigns to eliminate on-site sterilization, cleaning, and risk of cross contamination.
Configuring Your Modules7
Each module type or a combination of modules may offer a unique project advantage; however, considerable thought must be given to module size and configuration in concert with waterways and rail and road limits. Module types include:
- Truckable modules suited for complete modular process buildings. These have greater road travel flexibility.
- Skid-mounted process equipment modules
- Hybrid (super) process skid modules
- Plug-and-play pods that can be installed inside a building shell, e.g., cleanroom suites
Once the decision has been made to use modular fabrication, the next step in the journey is to consult with module designers and fabricators to provide the design intelligence to configure the modules while optimizing schedule, cost, and site logistics.
Shifting The Design Paradigm8
Design thinking has been around for nearly 50 years, but companies continue to struggle to realize value from design. Shifting the design paradigm by using some well-documented design approaches and team behaviors can deliver agility to a project team. Here are key shifts to make to optimize modularization:
- From traditional design phases (i.e., concept, basis of design, detailed design) to early design planning by building flexibility to manage design through the modular fabrication shop floor.
- From relying on narrow subject matter experts (SMEs) to using interdisciplinary designers and leveraging real-time digital design tools.
- From project functions to an integrated factory team, by adopting and implementing an early integrated project delivery (IPD) model.
- From prototyping once to a continuously improved and repeatable plant that lends itself to duplication and creates an organic environment for predictable results.
- From the project team to the C-suite, by implementing a governance model that establishes executive sponsorship and real partnerships across the enterprise and suppliers.
- From incremental to long-term business relationships, by focusing on operations life cycle value to strengthen innovation.
- From traditional RFP to an IPD model that can complement a target value design approach and incentive-based contract at the early project planning stage.
Off-site construction and modularization can facilitate the implementation of best practices in a controlled environment while improving delivery of key project fundamentals. While IPD can help define how the project will flow and lean manufacturing provides the means, TVD can be instrumental to set the right cost target; however, this requires a governance structure and a rigorous process. Do not ignore digitalization; developing a digital project execution plan is not far-fetched in this era, but its ultimate impact can be limited if we do not consider how it can best be deployed at the project, program, or enterprise level. Digital capability can add real value by tying together as-designed, as planned, and as-built information required to manage the asset through its operations and maintenance life cycle.
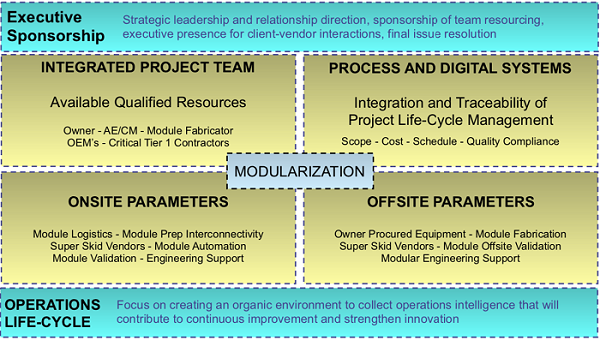
Figure 1: Modularization operating model and enablers
An operating model can enable the application of the modular strategy to the business or operation to represent how the project can be best organized to more efficiently and effectively deliver and execute on the plan.
Nontraditional construction costs for off-site module fabrication include logistics, transportation, staging, and large crane requirements not needed on stick-built sequential construction.
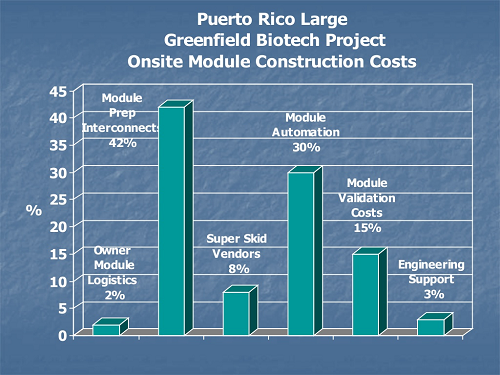
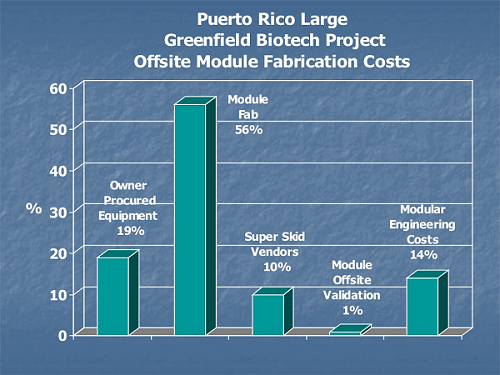
Figure 2: Comparison of on-site and off-site module fabrication costs9
Conclusion
Measurable benefits of off-site module construction are becoming more evident, and it is emerging as a viable method for delivering a greater degree of predictability. Furthermore, market constraints driven by shortages of craft labor, lagging productivity, project complexity, and competitive forces are contributing to biopharma’s move toward modularization. The Construction Users Roundtable (CURT), among other construction industry forums, recognizes the concept and is providing its members the balanced information they need to help them get started. The intersection of the biopharma company owner, the modular design-fabricator, the AE/CM, and OEMs requires strong governance, a clear team structure, and a rigorous workflow process.
Photo of prefabricated cleanroom POD courtesy of G-CON Manufacturing, Inc.
References:
- Research and Markets, Modular Facilities in Pharmaceutical/Biotechnology Industry, 2017-2030, October 2017.
- EPIC Process Systems, “The 5 Major Advantages Of Modular Process System Design,” https://www.epicmodularprocess.com/blog/advantages-of-modular-design.
- Biopharma International – “Modular Manufacturing Platforms for Biologics,” May 2015.
- The Voice (CURT), “Making the move to Offsite Construction and Modularization,” Winter 2019.
- Bioengineering & Translation Medicine - Evolving trends in mAb production processes, March 2017.
- E.S. Langer, et al., Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, 15th Edition. BioPlan Associates: Rockville, MD, April 2018.
- Hydrocarbon Processing, “Modularization: The key to success in today’s market,” December 2016
- McKinsey & Company, “More than a Feeling: Ten design practices to deliver business value,” December 2017.
- Abelardo (Abe) Perez, Jr., Modular Construction: An Economic Overview of Completed Projects
About the Author:
 Hector M. Samper is an independent consultant and strategic advisor at Global Strategic Sourcing Solutions (GSSS). He formed GSSS to provide business solutions to the biopharma sector, including services to transform and guide cross-functional business/procurement teams to generate maximum value throughout the operations lifecycle. Samper has over 30 years of experience in the capital expenditure and operations maintenance arena, including in a number of indirect spend categories across North America, Europe, and Latin America. His recent experience at Sanofi included creating and leading the Strategic Vendor Management Office for the Global IT function and heading the North America Operations and CapEx Procurement group. Prior to Sanofi, Samper worked for Amgen, Purdue Pharma, and Merck, where he held roles of increased responsibility in the global engineering, sourcing, and procurement organizations. He earned a bachelor’s degree in civil engineering from the New Jersey Institute of Technology. You can contact him at hmsamper@aol.com.
Hector M. Samper is an independent consultant and strategic advisor at Global Strategic Sourcing Solutions (GSSS). He formed GSSS to provide business solutions to the biopharma sector, including services to transform and guide cross-functional business/procurement teams to generate maximum value throughout the operations lifecycle. Samper has over 30 years of experience in the capital expenditure and operations maintenance arena, including in a number of indirect spend categories across North America, Europe, and Latin America. His recent experience at Sanofi included creating and leading the Strategic Vendor Management Office for the Global IT function and heading the North America Operations and CapEx Procurement group. Prior to Sanofi, Samper worked for Amgen, Purdue Pharma, and Merck, where he held roles of increased responsibility in the global engineering, sourcing, and procurement organizations. He earned a bachelor’s degree in civil engineering from the New Jersey Institute of Technology. You can contact him at hmsamper@aol.com.
