BD Vystra Disposable Pen For The Self-Injection Of Chronic Disease Medications
Source: BD Medical - Pharmaceutical Systems
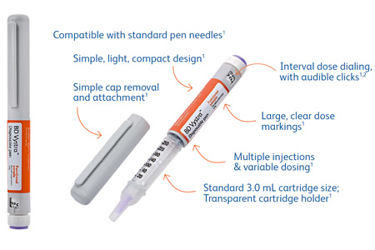
Designed for use with a wide range of drug therapies that require variable dosing:
- Adjustable features and clear dose markings to support self-injection and ease of use
- Patient Centric Design-Over 100 Patients, HCPs, & Experts informed the BD Vystra™ Disposable Pen injector design
- Comes with regulatory and technical documentation including but not limited to:
- Human Factors Engineering Report
- Validated platform Instructions for Use (IFU) available for reference
- Technical information to support Regulatory Submissions: Master File (MAF) for US Market and Technical Dossier (TD) for Key Global Markets
- R&D documentation to support development activities
access the Brochure!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Biosimilar Development? Subscribe today.
Subscribe to Biosimilar Development
X
Subscribe to Biosimilar Development
