Comparison Of Sterilization Methods: Vaporized Vs. Aerosolized Hydrogen Peroxide
By Sandeep Desai, director of facilities and engineering, Empower Pharmacy

Hydrogen peroxide (H2O2) has been one of the most frequently used chemical compounds for sterilization for decades. Its broad-spectrum antimicrobial properties and low-heat sterilization make it perfect for heat-liability equipment and spaces (among other properties). Hence, it is among the most optimal options for various sterilization scenarios.1 While most of its beneficial properties hold true in all scenarios, the efficacy, impact, and use cases may differ significantly based on the hydrogen peroxide sterilization method used. The two most common methods are vaporized hydrogen peroxide and aerosolized hydrogen peroxide. Vaporized hydrogen peroxide typically requires heat, while aerosolized hydrogen peroxide is typically generated using a mechanical atomizer. This differentiation of conversion (state) and dispersion method affects everything from cost, exposure time, and concentration to residual characteristics. Still, both methods have their uses. In this article, we will compare the two.
Method
Vaporized Hydrogen Peroxide Process
There are multiple vaporized hydrogen peroxide generation processes, with closed loop being one of the most common. The method may also be classified based on how the hydrogen peroxide solution is vaporized. Flash vaporization is a commonly used technique, but other techniques exist as well. In this process, a hydrogen peroxide solution of a concentration of 35% weight/weight (w/w) is typically used. Flash evaporation differs from typical thermal evaporation because heated hydrogen peroxide is de-pressurized by transporting it through a passage that gradually expands, causing part of the heated solution to instantly vaporize.
Figure 1: Visualization of the flash evaporation chamber heated hydrogen peroxide passes through for depressurization and flash evaporation2
A single decontamination cycle of the vaporized hydrogen peroxide process is completed in four phases.3
- The first step is stabilizing the temperature of the vaporized hydrogen peroxide generator/equipment and stabilizing/maintaining the desired humidity level in the chamber/space to be decontaminated.
- In the second step, the hydrogen peroxide is flash vaporized, and the vapor is pushed into the chamber/space until the necessary concentration of vapor is established in the chamber. The concentration level is correlated to the inactivation efficacy of the process and the chemical agent.
- The third step is introducing more vapor into the chamber to maintain the desired concentration.
- The fourth step is pushing clean air into the chamber to bring the hydrogen peroxide concentration down to acceptable levels. These levels may differ for a chamber dedicated to decontaminating medical equipment and a chamber/closed space like a room that is being disinfected.
It’s worth noting that the inactivation efficiency of the vaporized hydrogen peroxide sterilization process is highly influenced by concentration, but when it comes to the D value (decimal reduction time), which represents the time required to reduce the microbial population by 90% (or one log reduction), the humidity of the chamber also becomes a critical factor.
Higher humidity levels facilitate the condensation of hydrogen peroxide on surfaces. This increased condensation results in more effective contact between the H2O2 and microorganisms, which enhances its antimicrobial action. Hence, high humidity leads to lower D values at the same concentration compared to lower humidity, which leads to longer kill times and higher D values.4 However, it risks leaving residues that may need to be removed afterward. Wet VHP is less commonly used and generally avoided in environments where residue or moisture is a concern.
Aerosolized Hydrogen Peroxide Process
The aerosolized hydrogen peroxide process relies upon the aerosolization of less concentrated hydrogen peroxide solution. The concentration is typically between 5% and 7%.5 Sometimes, silver cations may be used to enhance the antimicrobial properties of the solution, which can impact both concentration and particle size generated by aerosolized hydrogen peroxide equipment. The concentration can increase (up to 12%, as per one source), and particle size may shrink from the usual 8 to 10 micrometers to 4 micrometers.6 Both the concentration and particle size are also influenced by the aerosolization mechanism, i.e., whether or not it’s augmented by plasma activation. Plasma activation is also more effective against a wider range of microorganisms.
A typical aerosolized hydrogen peroxide generator relies upon an atomizer to convert a hydrogen peroxide solution to mist/fog/aerosols. Some generators rely upon pressurized air to convert liquid solutions into aerosols (leveraging a shearing force to break the liquid solution into small droplets). The air also can disperse the mist. Other generators use an ultrasonic atomizer that produces aerosols by passing a high-frequency soundwave just below a thin surface of water, leveraging the energy of the soundwaves to convert water into small droplets. Its main benefit is uniform aerosol size. The mist can be dispersed through small fans incorporated in the aerosolized hydrogen peroxide generator.
In plasma-activated aerosolized hydrogen peroxide generators, the plasma generators are placed after the atomizer and activate the aerosols by passing them through an electrode-generated cold plasma field.
Comparison Of The Two Methods
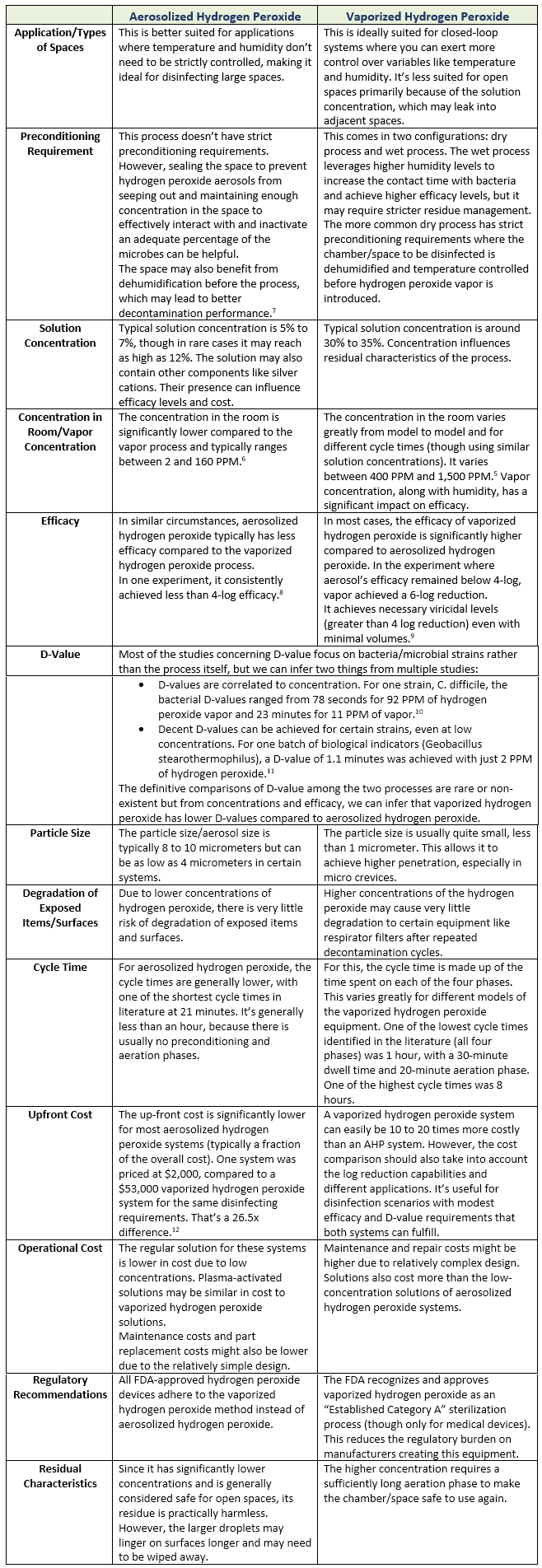
Conclusion
Vaporized hydrogen peroxide has a clear edge over aerosolized hydrogen peroxide in terms of efficacy and D-values. Aerosolized hydrogen peroxide has a significantly lower cost benchmark, and the equipment is easy to use and deploy. Both have different applications. However, for applications where the log reduction requirements are permissive enough to allow both processes, aerosolized hydrogen peroxide may be more effective. Where efficacy levels are the top priority, vaporized hydrogen peroxide should be preferred.
References
- M. S. Kyi, J. Holton and G. L. Ridgway, "Assessment of the efficacy of a low temperature hydrogen peroxide gas plasma sterilization system," Journal of Hospital Infection, 1995.
- I.F. McVey, F.J. Zelina, A.L. Hill, G.E. McDonnell, K.O. Williams, T. Mielnik, United States Patent US7157046B2, 2007.
- D. Watling, C. Ryle, M. Parks and M. Christopher, "Theoretical Analysis of the Condensation of Hydrogen Peroxide Gas and Water Vapour as Used in Surface Decontamination," PDA Journal of Pharmaceutical Science and Technology, pp. 291-299, 2002.
- B. Unger-Bimczok, V. Kottke, C. Hertel and J. Rauschnabel, "The Influence of Humidity, Hydrogen Peroxide Concentration, and Condensation on the Inactivation of Geobacillus stearothermophilus Spores with Hydrogen Peroxide Vapor," Journal of Pharmaceutical Innovation, p. 123–133, 2008.
- D. Berger, G. Gundermann, A. Sinha, M. Moroi, N. Goyal and A. Tsai, "Review of aerosolized hydrogen peroxide, vaporized hydrogen peroxide, and hydrogen peroxide gas plasma in the decontamination of filtering facepiece respirators," American Journal of Infection Control, p. 203–213, 2021.
- C. Freyssenet and S. Karlen, "Plasma-Activated Aerosolized Hydrogen Peroxide (aHP) in Surface Inactivation Procedures," Applied Biosafety, p. 10–19, 2019.
- Arunwuttipong, P. Jangtawee, V. Vchirawongkwin, W. Kangwansupamonkon, K. Asavanant and S. Ekgasit, "Public Buses Decontamination by Automated Hydrogen Peroxide Aerosolization System," Macedonian Journal of Medical Sciences, pp. 847-856, 2021.
- T. Fu, P. Gent and V. Kumar, "Efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems," Journal of Hospital Infection, pp. 199-205, 2012.
- S. M. Goyal, Y. Chander, S. Yezli and J. A. Otter, "Evaluating the virucidal efficacy of hydrogen peroxide vapour," Journal of Hospital Infection, 2014.
- D. J. Malik, C. M. Shaw, G. Shama, M. R. J. Clokie and C. D. Rielly, "An Investigation into the Inactivation Kinetics of Hydrogen Peroxide Vapor Against Clostridium difficile Endospores," Chemical Engineering Communications, pp. 1615-1624, 2016.
- D. Kümin, M. G. Albert and K. Summermatter, "Comparison and Validation of Three Fumigation Methods to Inactivate Foot-and-Mouth Disease Virus," Applied Biosafety, 2018.
- L. Mead, T. Mathison and A. M. Richards, "Decontamination of Geobacillus Stearothermophilus Using the Arca Aerosolized Hydrogen Peroxide Decontamination System," PLOS ONE, 2022.
About The Author:
Sandeep Desai is director of facilities and engineering at Empower Pharmacy. He has 20+ years of experience in pharmaceutical engineering, specializing in HVAC systems, water systems, and manufacturing process equipment. With years of experience in facility design, qualification, and process optimization, he has implemented advanced HVAC solutions to maintain classified environments and ensure regulatory compliance. Adjacent experience lies in designing, installing, and optimizing purified water, WFI, and clean steam systems to meet stringent pharmaceutical standards. He has led modifications and enhancements in filling lines, lyophilizers, autoclaves, and sterilization systems to improve efficiency, reliability, and product integrity. In previous roles, he has worked with OSD and ointment facilities.
