Considerations For Advancing A Lipid Nanoparticle Formulation To Clinical And Commercial Manufacturing
By Mahesh Karwa, PhD, Associate Director of Process Development, Teresa Demuth, PhD, Strategic Marketing Manager, and Gaëlle Béalle, PhD, Strategic Marketing Manager
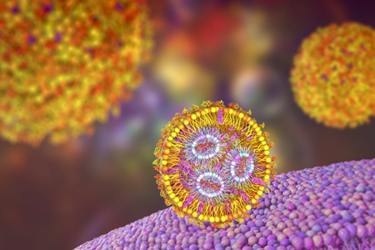
Various lipid-based systems can serve as carriers for delivering nucleic acids, including mRNA and plasmid DNA, for therapeutic and vaccination purposes. Following the success of COVID-19 vaccines, lipid nanoparticles (LNPs) have garnered significant interest as a robust and scalable non-viral system for gene delivery.
LNPs are structured with four categories of lipids, which are crucial excipients in the formulation of oligonucleotide-based therapeutics and vaccines. Ionizable lipids encapsulate nucleic acids and facilitate payload release. Polyethylene glycol (PEG) lipids enhance particle stability and extend circulation time. Meanwhile, cholesterol and helper lipids offer structural support to the particle and ensure the proper rigidity.
Selecting lipids with the appropriate quality impacts both the final LNP drug product and the LNP process. This white paper reviews critical lipid quality considerations and outlines the process requirements necessary for successful commercial-scale manufacturing.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Biosimilar Development? Subscribe today.
