COVID-19's Impact On Bioprocessing: Insights From Industry Leaders
By Ronald A. Rader, Eric S. Langer, and Kamna Jhamb, Ph.D., BioPlan Associates, Inc.
The COVID-19 pandemic is affecting nearly all global industries and social functions, including the biopharmaceutical  industry and bioprocessing. As part of our 17th Annual Report and Survey of Biopharmaceutical Manufacturing,1 in addition to the survey of 260+ biopharma manufacturers and suppliers, we interviewed 26 biopharma senior executives regarding how they are handling the crisis and its long-term impact on their operations. We found the pandemic has begun to catalyze and accelerate a number of significant changes, including many preexisting trends.
industry and bioprocessing. As part of our 17th Annual Report and Survey of Biopharmaceutical Manufacturing,1 in addition to the survey of 260+ biopharma manufacturers and suppliers, we interviewed 26 biopharma senior executives regarding how they are handling the crisis and its long-term impact on their operations. We found the pandemic has begun to catalyze and accelerate a number of significant changes, including many preexisting trends.
Even before the crisis, this essential industry had been undergoing significant, steady advances. Having seen healthy, consistent 12 percent annual growth1 for at least the past 20 years, the biopharmaceutical industry and its bioprocessing sector have matured relatively rapidly.
During this period of rapid growth, the industry has learned to be flexible and to adapt quickly to change. This adaptability is already helping the industry retool for rapid development and production of pandemic vaccines, therapeutics, equipment, and diagnostics, while also responding to changes that this worldwide mobilization of resources will bring to the industry.
Industry Views Of Long-term Effects
Some pandemic-related changes or trends with long-term strategic implications include:
- Bioprocessing will rapidly accelerate globally
- Supply chain strengthening
- Single-use systems
- Process intensification — doing bioprocessing faster, with smaller facilities and fewer staff
- Regionalization — more bioprocessing done more globally
- Outsourcing — more use of contract manufacturing organizations (CMOs)
- Staffing — expect hiring problems to accelerate
- Increased automation
Even before the current COVID-19 pandemic, all of these trends were evident. Based on our current research, we found changes being undertaken resulting from the pandemic that are impacting near-term industry dynamics. But the future-casting offered by industry executives presented some significant, long-term transformational changes that are likely to occur, as well.

Figure 1: Long-term effects of COVID-19 on bioprocessing1
Near-term Impacts
Overall, in the near term there is increasing R&D and manufacturing and shifting of resources toward pandemic response. The bioprocessing sector in the near term is experiencing operational and staffing problems, often related to increased activity or adapting to social distancing. Most bioprocessing-related industrial activities are considered essential and are continuing largely unaffected in terms of operations and output, while many are planning to ramp up R&D and manufacturing. While there are many near-term changes in on-site staff management, broader business plans are generally not affected in the near term.
As expected, many companies, including developers and suppliers of hardware and services, are increasing their pandemic-related R&D and manufacturing. Suppliers are anticipating and planning for increased business as companies and governments start to rapidly develop, test, and deploy pandemic-related vaccines, therapeutics, and diagnostics. There has also been an increase in demand for short-term stocking up on supplies, with many facilities planning to store more supplies on-site and seek backup sources for supplies and services.
Biopharmaceutical-related facilities, equipment suppliers, CMOs, CROs, and R&D/developer companies are considered essential and have continued operations with only minimal initial disruptions, most of which have been associated with management of operations staff during this crisis. The general expectation is that there will be relatively minimal adverse near-term economic impact from the pandemic and its aftermath on the bioprocessing sector. Most companies are continuing with their long-term capital-intensive expansions and mergers/acquisitions, with many accelerating their plans.

CDMOs/CROs may face challenges as some prospective non-pandemic-related projects are postponed or canceled, while those with viral vaccine-related services see increased business. This may presage mid-term challenges for the sales pipeline of some service suppliers, particularly those not supporting pandemic-related R&D or manufacturing. But with bioprocessing CMOs overall having as much as a year’s backlog of projects, CMOs are insulated from many of the short-term revenue disruptions.
Fears To Be Addressed
The interview results below report some of the key fears, options for their resolution, and the expected “new normal” for the bioprocessing industry that were cited by the interviewees. The number one fear reported by both suppliers and biopharma was of shortages of single-use and other critical supplies, noted by 70 percent and 75 percent, respectively. Already there are long wait times for many single-use supplies, and these will likely lengthen for non-pandemic response-related projects.
Following the fear of single-use shortages were the problems associated with prioritization of non-Covid projects and the need to ramp up manufacturing (for suppliers).
In the future, respondents, both biopharma company and suppliers, expect to see a new normal, with collaborations among stakeholders likely to increase. Today, as the world works through this crisis, collaborations are seen as indispensable. This includes cross-company, cross-industry, with governments, and multinationally. There is a clear sense that we are all in this together, and that the current situation may well be defining our relationships in the future. If any positive outcome results from this pandemic, it may be the recognition that working together is essential today, and this will likely carry on into the future.
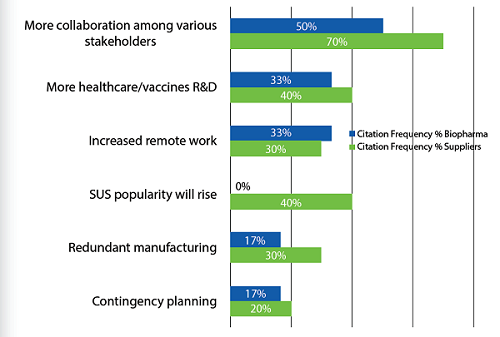
Figure 2: “New normal” in bioprocessing post-COVID-19
Summary Of Effects On The Industry
The industry is likely heading toward the following as a result of the COVID-19 crisis.
- More Supply Chain Oversight: The pandemic will result in more product, supply, and service companies exercising more control over their supply chains, including reaching further upstream to more suppliers.
- More Facilities: Even at this early point in the crisis, we see that there will easily be $15 to $20 billion or more invested in new pandemic-related vaccines and therapeutics. Likely half or more will go to new manufacturing facilities.
- More Bioprocessing: Bioprocessing and related supplies and support activities will increase. This will include significant rapid increases in pandemic- and infectious disease-related R&D and manufacturing by companies and governments.
- Prioritization of Supplies and Services: Prioritization of outsourced projects and supplies orders will be part of the new normal after the pandemic subsides.
- Single-use vs. Stainless Steel: There will be more single-use systems (SUS) in new process lines and facilities.
- More Modular facilities: Many new pandemic-related vaccine and biotherapeutic facilities will likely be modular. This trend has been accelerating for at least five years; the crisis will precipitate more rapid expansion.
- Single-use (and Other) Shortages: Because pandemic-related bioprocessing process lines and facilities are or will generally be SUS-based, suppliers are concerned about future shortages.
- More Regionalization/Decentralization: More new facilities, including vaccine-related and for bioprocessing supplies, will be located internationally, with emphasis on regional manufacturing. This is now seen as essential to deal with any future pandemics.
- Faster R&D and Speed-to-Market: The pandemic is showing that (bio)pharmaceutical R&D needs to be more streamlined.
- More Collaboration: Those in the industry recognize the need for more collaboration and communication among bioprocessing professionals at all levels.
- Better Inventory Management: The need for better inventory management is becoming obvious. Before the crisis, delays in obtaining required equipment for biologics production were a challenge. Today, avoiding delays is a critical mission.
- More Investment: The private and public sector will increase investments in pandemic- and other infectious disease-related R&D and manufacturing. Already, BioPlan is tracking well over $10 billion in projected new pandemic-related vaccines and therapeutics development efforts.
- More Staffing Difficulties: Chronic staffing challenges have plagued the industry for 15 years. These will be exacerbated by expected increases in pandemic-related products.
- Shipping Delays: Adverse effects on shipping are being already experienced by many suppliers.
- More Automation: Related to the staffing difficulties, the desire to decrease on-site staff, and process intensification, there will be increasing investments in automation.
Changes in the bio/pharmaceutical industry are being accelerated and catalyzed by the responses to the current COVID-19 pandemic. Although suppliers have proven themselves rather robust in their dealing with the pandemic, and business is continuing generally uninterrupted, there will be significant changes. Ongoing and accelerating trends will result in changes including improved strategies to moderate future pandemics and supply disruptions, development of collaborative relationships in R&D and among suppliers, and the implementation of more rapid, flexible, and modular production processes to speed products to the market. Along with rapid expansion in bioprocessing capacity, these changes will affect manufacturing long after the current crisis resolves.
References:
- 17th Annual Report and Survey of Biopharmaceutical Manufacturing and Production, BioPlan Associates, Rockville, MD April 2020, 450 pages, www.bioplanassociates.com/17th.
About the Authors:
 Ronald A. Rader is senior director of technical research at BioPlan Associates, Inc. He has 35+ years’ experience as a biotechnology and pharmaceutical — particularly biopharmaceutical — information specialist, analyst, and publisher, and has been responsible for the Antiviral Agents Bulletin periodical; Federal Bio-Technology Transfer Directory; BIOPHARMA: Biopharmaceutical Products in the U.S. and European Market (www.biopharma.com); and the Biosimilars/Biobetters Pipeline Directory (www.biosimilarspipeline.com). You can reach him at rrader@bioplanassociates.com or 301-921-5979.
Ronald A. Rader is senior director of technical research at BioPlan Associates, Inc. He has 35+ years’ experience as a biotechnology and pharmaceutical — particularly biopharmaceutical — information specialist, analyst, and publisher, and has been responsible for the Antiviral Agents Bulletin periodical; Federal Bio-Technology Transfer Directory; BIOPHARMA: Biopharmaceutical Products in the U.S. and European Market (www.biopharma.com); and the Biosimilars/Biobetters Pipeline Directory (www.biosimilarspipeline.com). You can reach him at rrader@bioplanassociates.com or 301-921-5979.
 Eric S. Langer is president and managing partner at BioPlan Associates, Inc., a biotechnology and life sciences marketing research and publishing firm established in Rockville, MD, in 1989. He is editor of numerous studies, including Biopharmaceutical Technology in China, Advances in Large-scale Biopharmaceutical Manufacturing, and many other industry reports. You can reach him at elanger@bioplanassociates.com or 301-921-5979.
Eric S. Langer is president and managing partner at BioPlan Associates, Inc., a biotechnology and life sciences marketing research and publishing firm established in Rockville, MD, in 1989. He is editor of numerous studies, including Biopharmaceutical Technology in China, Advances in Large-scale Biopharmaceutical Manufacturing, and many other industry reports. You can reach him at elanger@bioplanassociates.com or 301-921-5979.
 Kamna Jhamb, Ph.D., is director of technical research at BioPlan Associates. With a Ph.D. in biotechnology/microbiology, she has extensive experience working in several key industry segments, including at the Lawrence Berkeley National Lab (LBNL) and elsewhere. Her expertise lies in primary and secondary research and market analysis of healthcare and biopharma segments. She has contributed to international publications and journals, in-depth reports, analyses, and strategic white papers.
Kamna Jhamb, Ph.D., is director of technical research at BioPlan Associates. With a Ph.D. in biotechnology/microbiology, she has extensive experience working in several key industry segments, including at the Lawrence Berkeley National Lab (LBNL) and elsewhere. Her expertise lies in primary and secondary research and market analysis of healthcare and biopharma segments. She has contributed to international publications and journals, in-depth reports, analyses, and strategic white papers.
