Development Of A Two-Column Manufacturing Process For Adenovirus
Large-scale downstream processing of viruses for clinical applications poses challenges different from those for many other biotherapeutics. Adenovirus vectors are effective tools for the transfer of genetic material into mammalian cells. They offer several advantages, including the capacity to accommodate up to 37 kb of foreign genetic material, very high infection efficiency, ability to infect a wide variety of both dividing and nondividing cell types, lack of integration into the host chromosome, and availability of production systems capable of generating high virus titers. These and other qualities have led to adenoviruses being the most used gene transfer vectors in experimental therapies, accounting for 25% of all gene therapy trials. As of 2014, they had been used in almost 500 clinical trials.
 Large-scale downstream processing of viruses for clinical applications poses challenges that arise, in part, from the viruses’ large size and complexity. In the case of adenovirus, one intact virus particle (vp) contains more than 2,700 protein subunits, with a mass of approximately 165 MDa, and a diameter of approximately 0.1 μm. The complexity of the particle gives rise to thousands of charge variants, making it difficult to establish well-defined binding and elution conditions on charged separation resin. In addition, adenoviruses tend to be acid labile, which further increases the complexity of process development.
Large-scale downstream processing of viruses for clinical applications poses challenges that arise, in part, from the viruses’ large size and complexity. In the case of adenovirus, one intact virus particle (vp) contains more than 2,700 protein subunits, with a mass of approximately 165 MDa, and a diameter of approximately 0.1 μm. The complexity of the particle gives rise to thousands of charge variants, making it difficult to establish well-defined binding and elution conditions on charged separation resin. In addition, adenoviruses tend to be acid labile, which further increases the complexity of process development.
A two-column efficient purification process was developed for a recombinant adenovirus after screening five chromatography resins (Table 1 and Table 2). The final process yields an active, concentrated virus product with purity, HCP levels, and DNA contamination comparable to clinical-grade products. The process is readily scalable and is sufficiently simple, rapid, and efficient to be used for the production of clinical-grade viral vectors.
Click Here to Learn More About Which Resin is Right for You
Process Development
Mass Capture
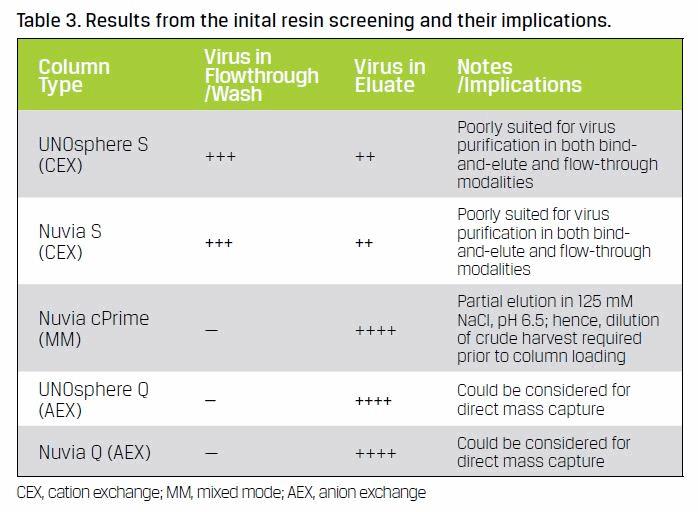 Initial screening showed that of the five resins selected three resins had potential for use in a mass capture process — Nuvia cPrime (hydrophobic cation exchange), UNOsphere™ Q (anion exchange), and Nuvia Q (anion exchange) — since the virus was not detected in either the flow-through or wash fractions (Table 3). Nuvia cPrime was selected for capturing the adenovirus as it offered the best clearance of feedstream contaminants. Eluate from this hydrophobic cation exchange resin could be loaded onto the subsequent column following a simple pH adjustment. Fouling of anion exchange chromatography resins in process manufacturing (Close et al. 2013, Drevin et al. 1989) and difficulties in their regeneration (Ng and McLaughlin 2007) have been reported due to the binding of excessive amount of impurities and ineffective cleaning. Therefore, the Q resins are more suitable for polishing purification. Between the two strong anion exchange resins, Nuvia Q was chosen for its higher binding capacity for target virus even in the presence of high NaCl concentrations.
Initial screening showed that of the five resins selected three resins had potential for use in a mass capture process — Nuvia cPrime (hydrophobic cation exchange), UNOsphere™ Q (anion exchange), and Nuvia Q (anion exchange) — since the virus was not detected in either the flow-through or wash fractions (Table 3). Nuvia cPrime was selected for capturing the adenovirus as it offered the best clearance of feedstream contaminants. Eluate from this hydrophobic cation exchange resin could be loaded onto the subsequent column following a simple pH adjustment. Fouling of anion exchange chromatography resins in process manufacturing (Close et al. 2013, Drevin et al. 1989) and difficulties in their regeneration (Ng and McLaughlin 2007) have been reported due to the binding of excessive amount of impurities and ineffective cleaning. Therefore, the Q resins are more suitable for polishing purification. Between the two strong anion exchange resins, Nuvia Q was chosen for its higher binding capacity for target virus even in the presence of high NaCl concentrations.
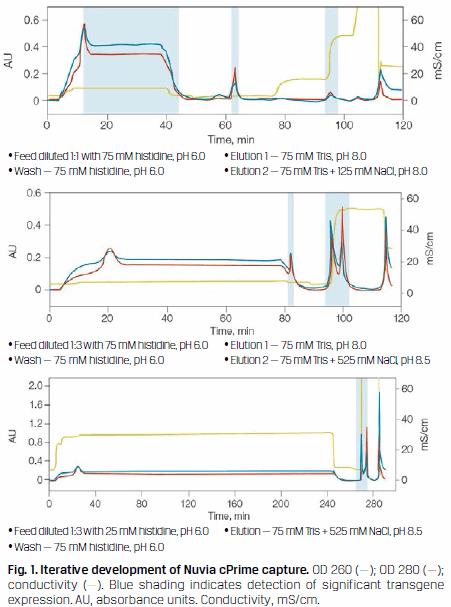 A nuclease digestion was introduced prior to column loading to aid in nucleic acid clearance. Initial mass capture experiments were focused on reducing feedstream volumes and recovering virus. The chromatograms in Figure 1 illustrate the early progression of these experiments and indicate when significant transgene expression was detected.
A nuclease digestion was introduced prior to column loading to aid in nucleic acid clearance. Initial mass capture experiments were focused on reducing feedstream volumes and recovering virus. The chromatograms in Figure 1 illustrate the early progression of these experiments and indicate when significant transgene expression was detected.
Anion Exchange Chromatography
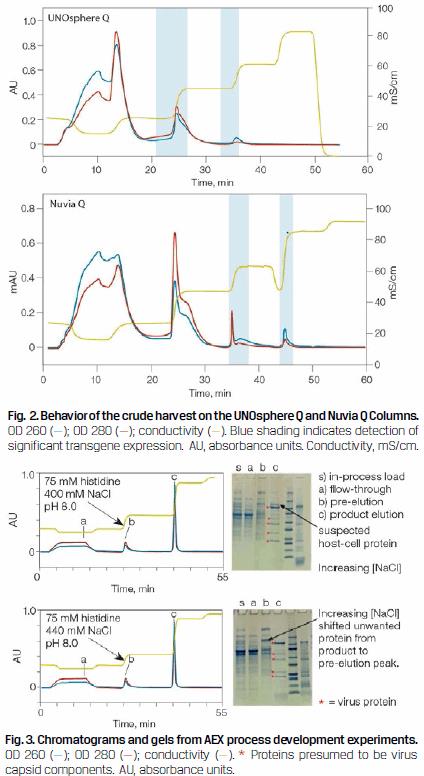
Of the two anion exchange resins, UNOsphere Q and Nuvia Q, Nuvia Q was selected because it could adsorb virus at higher NaCI concentrations (Figure 2). It was therefore easier to work with downstream of the Nuvia cPrime capture step, where the eluate had an NaCI concentration of approximately 500 mM going on to the anion exchange column.
The anion exchange experiments with Nuvia Q were focused on attaining high product purity (Figure 3).
As shown in Figure 3, increasing the pre-elution wash NaCl concentration from 400 to 440 mM prevented a suspected HCP contaminant from eluting with the product. Therefore, it would be possible to further improve the efficiency of this chromatography step by directly equilibrating the Nuvia Q Column with 75 mM Tris + 440 mM NaCl, pH 8.0.

FINAL PROCESS
Mixed-Mode Chromatography
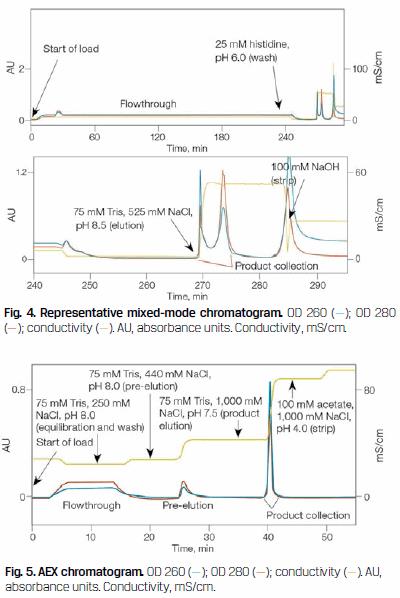
Initial capture was accomplished using Nuvia cPrime Mixed-Mode Resin (Figure 4). This portion of the process achieved a ten-fold reduction in processing volume and a significant reduction in feedstream contaminants (Figure 6, lanes 2–4).
Anion Exchange Chromatography
Final virus purification was accomplished using Nuvia Q Resin (Figure 5). This portion of the process achieved an additional two-fold reduction of product volume along with a significant improvement in product purity (Figure 6, lanes 4–7). Following this operation, nonvirus proteins were no longer evident by SDS-PAGE (Figure 6, lane 7).
Analysis of In-Process and Final Product
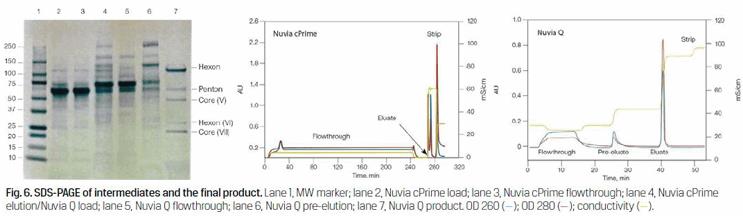
 SDS-PAGE was used for analysis to visualize the progressive reduction of contaminating proteins at each step of the purification process (Figure 6). Cell culture media components such as albumin and transferrin are clearly evident in the virus culture harvest, that is, the sample loaded onto the Nuvia cPrime Column (Figure 6, lane 2). These proteins are effectively separated from the viral protein components, as they are highly visible in the flow-through fraction from this column, under the current chromatography conditions (Figure 6, lane 3). Significantly fewer contaminants are seen in the sample loaded onto the Nuvia Q Column (Figure 6, lane 4). During the anion exchange process, the virus bound to the resin while contaminants either flowed through the column (Figure 7, lane 5) or were pre-eluted (Figure 6, lane 6). The five most prominent viral proteins — hexon, penton, core (V), hexon (VI), and core (VII) — are readily visible in the final purified product (Figure 6, lane 7).
SDS-PAGE was used for analysis to visualize the progressive reduction of contaminating proteins at each step of the purification process (Figure 6). Cell culture media components such as albumin and transferrin are clearly evident in the virus culture harvest, that is, the sample loaded onto the Nuvia cPrime Column (Figure 6, lane 2). These proteins are effectively separated from the viral protein components, as they are highly visible in the flow-through fraction from this column, under the current chromatography conditions (Figure 6, lane 3). Significantly fewer contaminants are seen in the sample loaded onto the Nuvia Q Column (Figure 6, lane 4). During the anion exchange process, the virus bound to the resin while contaminants either flowed through the column (Figure 7, lane 5) or were pre-eluted (Figure 6, lane 6). The five most prominent viral proteins — hexon, penton, core (V), hexon (VI), and core (VII) — are readily visible in the final purified product (Figure 6, lane 7).
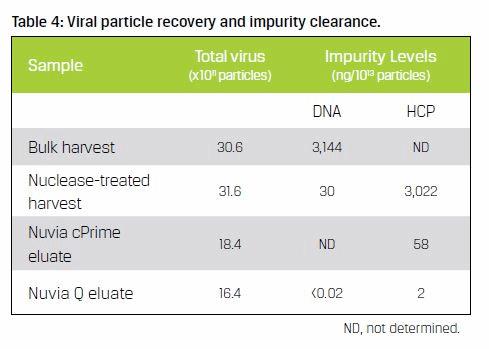
Virus concentration and DNA levels were evaluated at select points along the downstream process (Table 4). The data demonstrates an overall recovery of virus particles of approximately 54% ng/1013, with DNA levels below detection and HCP at 2 ng/1010 particles. These values are well within current guidelines for clinical and perhaps commercial use.
Summary
The final process yields an active, concentrated virus product with purity, HCP, and DNA levels comparable to clinical-grade products. While the purification methods presented here were developed using the Ad5-E1+GFP model virus, they are expected to be applicable to recombinant adenoviruses in general, and to constructs derived from serotype 5 viruses in particular. The process is readily scalable and uses procedures and reagents compatible with cGMPs. Also, it is sufficiently simple, rapid, and efficient to be used for the production of clinical-grade virus-based gene therapy products and vaccines.
References
Close, E.J. et al. (2013). Fouling of an anion exchange chromatography operation in a monoclonal antibody process: Visualization and kinetic studies. Biotechnol Bioeng 110, 2,425–2,435.
Drevin, I. et al. (1989). Column performance of Q-Sepharose HP in analytical- and preparative-scale chromatography. J Chromatogr A 477, 337–344.
Ng, P.K. and McLaughlin, V. (2007). Regeneration studies of anion-exchange chromatography resins. www.bioprocessintl.com/wp-content/uploads/bpi-content/070505ar06_77602a.pdf, accessed June 9, 2016.

