Integrated Systems For Sophisticated Drug Device Combination Products
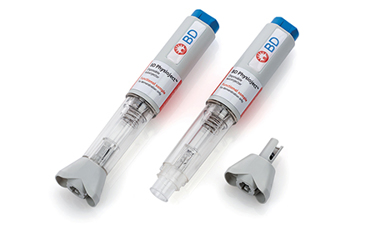
BD’s long history and experience in combination products has allowed it to develop deep domain knowledge to support the development of more robust, well-designed systems. Pharma company partners reduce the risk by selecting an integrated system of multiple components that work together to deliver the drug formulation safely and effectively.
In this interview, Dr. Alice Madden, Associate Director Regulatory Affairs at BD Medical – Pharmaceutical Systems and Lionel Maritan, Associate Director Research & Development at BD, discuss with detailed expert knowledge the benefits of using integrated systems for sophisticated drug device combination products with multiple device subsystems, and the crucial role that BD can play as systems integrator, delivering manifold advantages to its clients.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Biosimilar Development? Subscribe today.
