ISO 9001:2015 vs ISO 9001:2008 — What Has Changed? (Infographic)
The ISO 13485 standard, which outlines requirements for quality management systems (QMS) for medical devices, was originally written based on ISO 9001, and it has followed the general principles of ISO 9001 ever since.
A revised version of ISO 13485 will be published in the near future, and it will again follow ISO 9001 — specifically the ISO 9001:2015 edition that was just published in September.
The infographic below provides a detailed overview of the main changes in ISO 9001, together with the timing for transitions and all crucial information. Familiarizing yourself with these changes will help you prepare for the forthcoming revisions to ISO 13495.
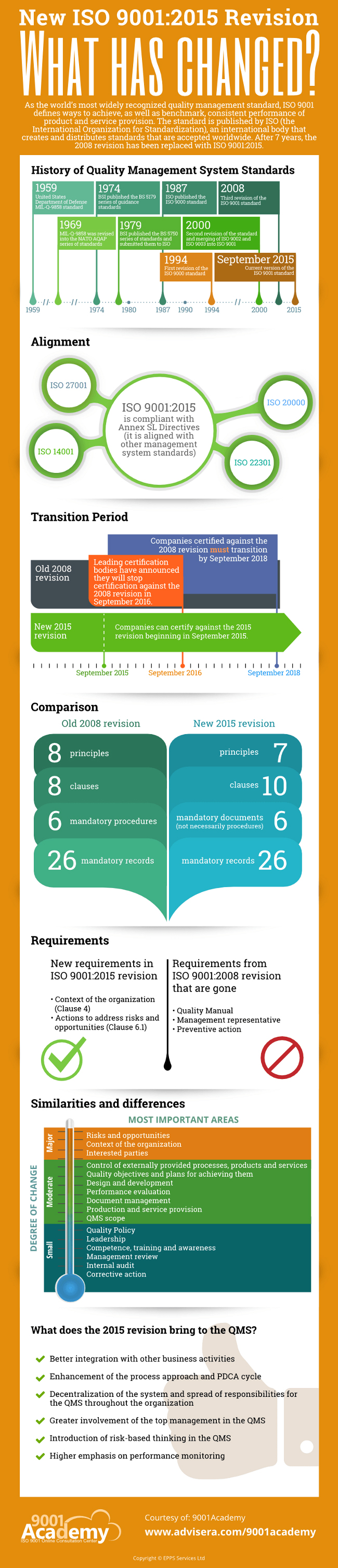
For more useful information on the changes in ISO 9001:2015, visit 9001Academy’s ISO 9001:2015 Revision Knowledge Base.
