White Paper: Process Analytical Technology (PAT) Using Next Generation Raman For Real-Time Bioprocess Monitoring
By Hemant Garg, Product Manager Analytics and Kevin Grollier, PAT Implementation and Support Engineer
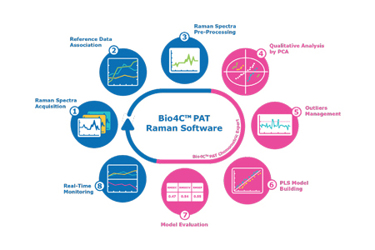
Process analytical technologies (PAT) and quality by design (QbD) guidelines published by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) reflect the concept that quality cannot be tested into a product but must be deployed throughout the life of a product. The facility of the future is one in which PAT, software automation, and analytics improve product quality, time to market and reduce manufacturing costs.
In this white paper, we describe applications and share case studies using Raman technology for real-time, inline monitoring of cell culture process parameters.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Biosimilar Development? Subscribe today.
