Shared Qualification Audits: A Win-Win-Win Approach
By Enith Morillo

In November 2021, the FDA published an update to the Resiliency Roadmap for FDA Inspectional Oversight, sharing the agency’s success in grappling with the impact of COVID-19 on domestic and foreign inspections and its effective use of remote interactive evaluations to support surveillance activities.
Throughout the pandemic, the agency has demonstrated more than ever the value of using a risk-based approach to inspectional activities, which must be embraced and mirrored in industry when it comes to supplier and vendor audits, especially by early-stage pharmaceutical companies.
Are Qualification Audits Required For Phase 1?
Qualification audits are not explicitly required as per the FDA’s cGMP guidance for Phase 1 investigational drugs but are generally considered a best practice in the industry. For early-stage pharmaceutical companies, qualification audits are the outset of GxP activities and are carried out prior to the start of Phase 1 clinical studies. Generally, sponsors will perform qualification audits of the CDMOs and CROs they intend to work with. However, unlike Big Pharma, where qualification audits are used as part of the vetting and selection process, qualification audits for early-stage companies are conducted post selection and contract negotiation and are used to validate the selection made and evaluate the compliance of the vendor’s quality system against regulatory requirements and industry standards. These audits are also used to establish rapport with the vendor and discuss project-specific requirements and how these are to be executed as per the vendor’s systems, processes, and controls.
It is common, and value-added, for these audits to be performed by sponsors’ quality assurance consultants, who are also involved in the day-to-day quality and compliance oversight of the vendor. When the auditor is familiar not only with the vendor’s facility but with its staff and procedures, the business relationship is furthered and efficiencies are gained.
Why Audit?
Sponsors strive to work with CDMOs that specialize in early-stage development and manufacture for delivery of cGMP drug substance, intermediates, and drug products in record time. These CDMOs offer the technical know-how, flexibility, and speed needed for the early stage paired with a phase-appropriate quality management system that can support the changes, deviations, and optimization expected when non-validated processes are scaled up from the lab to the plant.
It is noteworthy to mention that CDMOs that specialize in early stage and do not manufacture commercial products are exempt from registering with the FDA as per 21 CFR 207.13(e) and are therefore not subject to regulatory inspections on a regular basis.
Without regulatory oversight, risk emerges, making it imperative for the sponsor to evaluate the CDMO’s compliance and ensure adequate systems and procedures are in place for the performance of the contracted services. Thus, qualification audits become an integral part of the sponsor’s vendor oversight and the CDMO’s continuous improvement programs.
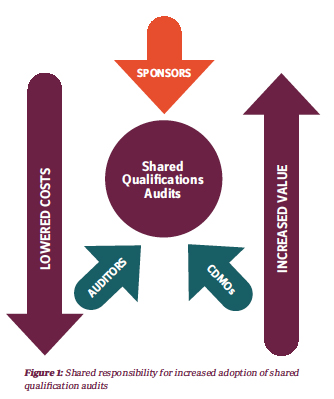
Shared Qualification Audits Are a Win For All Parties
Auditors may at times be asked by more than one sponsor to perform qualification audits of the same CDMO. Unlike requalification audits, where the focus is to verify product-specific requirements are being met and performance of contracted services is in line with the quality agreement and statement of work, qualification audits are generic and lend themselves to being “shared” by sponsors.
Shared qualification audits provide a cost-saving alternative for sponsors by opening the possibility of the splitting of expenses associated with the audit, and for CDMOs by reducing the resource-intensive cost of hosting multiple audits. Depending on the size of the CDMO, resources used to host and manage sponsor audits may not be dedicated, which can take a toll on the availability of quality assurance staff and other functional heads to focus on operational needs and priorities. By establishing best practices for the sharing of audits, the sponsors, the CDMOs, and the auditors can position themselves for successful and more rapid adoption of this value-added trend.
Shared Audits Are Currently Auditor-Driven
Once the need for a qualification audit is identified, the auditor can generally determine if the opportunity exists for the audit to be shared among sponsors. Key factors to be considered are the scope of services to be covered by the audit, the standards being audited against, and when the audit needs to be performed.
If all factors support the opportunity for sharing the qualification audit, the next step is for the auditor to confidentially approach the parties involved and inquire if there is interest in pursuing this alternative. It is recommended they approach sponsors first, separately, and explain how shared audits work and the benefits they provide. Once sponsors are comfortable and in agreement with pursuing a shared audit, the next step is to contact the audit host and discuss the logistics. It is important to ensure the audit host is amenable to accommodating a shared audit and agrees on specifics related to issuance of each sponsor’s audit report, findings, and requests for sponsor-specific responses, to maintain confidentiality throughout. In my years of experience as an auditor, CDMO audit hosts are generally familiar with and welcoming of shared audits and are agreeable to host these across two or more sponsors, where appropriate.
Removing Barriers To Increased Adoption
Over a decade ago, a somewhat similar approach was explored for excipient manufacturers’ audits, given their extensive use across the industry. Sizable efforts were made to establish and harmonize excipients’ GMPs and make a case for “purchasable” audit reports of excipient manufacturers. However, this did not catch on in the industry as expected.
Although shared audits are becoming more prevalent since the onset of the COVID-19 pandemic (given the added flexibility that virtual and hybrid audits bring to the audit table), the adoption of this trend in the pharmaceutical industry has been slow and is far from where it could be. For CDMOs that host anywhere from 50 to 100+ audits per year, a handful of shared audits does not drastically impact the extensive resources required to manage multiple audits on a weekly basis. The increase in adoption of shared audits is a shared responsibility that reduces auditing costs and increases value.
For starters, CDMOs must take a more active role in championing the feasibility and benefits of shared audits, endorsing and proposing these where appropriate, while exploring suitable avenues for the sharing of audit information. Their amenability to open their doors to auditors representing multiple sponsors must be recognized, as well as their interest in pursuing creative arrangements with independent auditors for generic qualification audits.
In addition, sponsors can support the adoption of shared qualification audits by educating themselves on what these are and being open to the benefits they provide, while being reassured that confidentiality is at the forefront of every auditor’s professional ethic.
Lastly, auditors must continue to spearhead the adoption of shared qualification audits where possible. By liaising between CDMOs and sponsors, auditors are in the privileged position to continue to develop best practices and increase the visibility of these audits and the value-add they provide to early- stage pharmas and the industry at large.
 About The Author
About The Author
Enith Morillo, M. SC., is the founder, president, and principal consultant of Cadoret Global, a quality and compliance consulting firm that specializes in supporting virtual, early stage, and small pharmaceuticals in taking their investigational drug through development and into Phase 1-2 clinical trials.
