The Importance Of Strategic Alliance Partners In Biopharmaceuticals
By Maria Barajas

Biotherapeutics are one of the fastest-growing sectors of the pharmaceutical industry. Between 2011 and 2016, revenue from biologics increased 70 percent to $232 billion. Industry analysts predict that number will continue to grow as more biologics and biosimilars enter the market and pharmaceutical companies across the globe build out their biotherapeutics pipelines. This rapid growth has sparked both challenges and opportunities for biopharmaceutical companies and their contract manufacturing partners as they seek to pair these novel therapies with innovative drug delivery systems.
Challenges of Strategic Alliances:
Many injectable biologics and biosimilars are coming onto the market as combination products – drug products paired with delivery devices – making it critical for pharmaceutical companies to pay close attention to the design, function and efficacy of integrated delivery systems. As such, biopharmaceutical companies are relying on drug delivery technology partners to provide containment, delivery and contract manufacturing expertise so that they can focus on developing these important drugs.
Strong collaboration between biopharmaceutical companies and their delivery device developer/manufacturer partners is key to delivering innovative, patient-centric delivery systems. Yet, these collaborations can be difficult to execute successfully. Any time multiple teams come together to work on a project there are bound to be challenges. These challenges are often compounded when the teams come from separate companies, each with their own culture, hierarchies and ways of communicating.
Dysfunction among collaborative parties is common and often rooted in one or more of these issues:
- Misaligned goals, processes and expectations
- Lack of accountability/responsibility
- Slow or lack of decision making
- Poor communication and transparency of information
- Blaming and finger pointing – little problem solving
These issues can lead to missed deadlines and/or poor quality products. With patient safety on the line, it’s critical that the teams get collaboration right. Culture is key to this success. Culture is what steers employee behaviors and motivation. A strong culture has the ability to drive people across teams to take pride and accountability in their work and focus on delivering value to customers.
To enable a culture of continuous improvement it’s important to outline guiding principles. These principles are the anchors that connect behaviors to desired results. They should be specific and meaningful to the team and map back to the overarching goals. For example, an organization that seeks to create a nurturing environment might set “Respect Every Individual” as a guiding principle and then outline a set of processes and protocols that enable a safe environment for sharing ideas. When teams align and adhere to a core set of guiding principles they naturally engender a stronger cultural affiliation and a more unified alliance.
Elements of Successful Alliances
With all of these challenges, how can biopharmaceutical companies and their drug delivery technology providers build a collaborative, integrated team? First, it’s important to align the collective team on a single purpose: serving patients. A focus on patients should guide everything the team does. Throughout the project, continually ask the question, “What is in the best interest of patients?”
Next, it’s important to establish some common guidelines and processes:
- Leadership – Effective leadership sets the tone for any successful collaboration. It’s important to build alignment and partnership among corporate leaders at each company by identifying and empowering co-executive sponsors to help guide the project from start to finish. Additionally, from the leadership team on down, establish single points of contact by function so the team is aware of resources and responsibilities.
- Goal Setting – Establish a common goal that is specific to the project, actionable and measurable. Ensure the goal is clearly communicated to the team and that the various facets of the project plan map back to the goal. Once the goal is set, take time to discuss and develop a shared vision for “what does good look like?” Have the team brainstorm their ideas and create specific statements for how they’ll achieve that vision and address and overcome potential problems along the way.
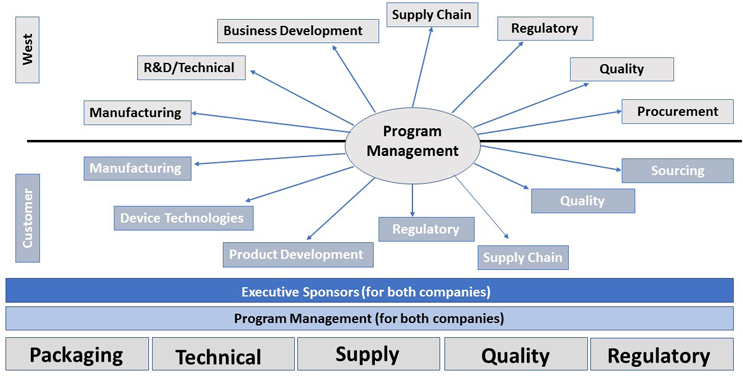
- Program Management Execution – The ability to achieve project results on time and within budget requires a special combination of leadership and process effectiveness. It is helpful to establish and agree upon an engagement model – i.e. a pathway to ensure alignment, focus, accountability and flawless execution (Figure 1).
- Accountability – Accountability is key throughout the entire project. At the outset, link goals and success factors to measurable activities by team members. In particular, make the team leads accountable for solutions in the best interest of patients and both companies. As the project begins – and throughout the various phases – ensure that teams are empowered to be successful. Finally, at the project conclusion, analyze how the teams fared in meeting their goals.
- Decision Making & Escalation – Clarity of decision making between companies and functions is key to keeping the project on track and executing against the goal. There are three primary roles in this process, each of which plays a critical function in the project’s success:
- Decision Maker – Staff members who are responsible and accountable for making decisions at the right level in both companies.
- Advisor – Staff members who provide advice to help make the decision.
- Informed – Staff members who are informed of and/or implement the decision
Everyone must work together collaboratively to ensure decisions are made and acted upon in accordance with the overarching goal. When decisions cannot be reached, the joint leads must escalate the issue via a defined pathway and in a timely manner.
- Ground Rules – Rule making and enforcement are important to any collaborative project, but they aren’t effective unless everyone agrees to abide by them. Before the project kicks off, take time to discuss and enact common ground rules across the collective team. Ground rules should be specific to the project and team. They can comprise a mix of high level and more tactical rules, such as:
- Always welcome new ideas
- Confirm all agreements in writing
- Surface issues immediately
- Look forward and not backwards
- Communicate decisions in a synchronous and timely manner
- Zero tolerance for negative behavior
Conclusion
Today’s drug delivery systems are complex pieces of technology that require close teamwork between biopharmaceutical companies and their drug delivery technology partners to develop innovative platforms that can be safely and efficiently used by patients themselves. Instead of operating as separate entities developing individual products, the parties must collaborate early and often to ensure self-administered therapies are safe, easy to use and effective. Creating an integrated, single team consisting of members from both parties with co-executive sponsorship and overarching program management leadership can enable the successful development and launch of combination products.
While there are many challenges to building a successful cross-business team, clearly defining and establishing goals and processes can help ensure a foundation of trust that empowers the team to deliver the best quality work. Key to the project’s success is collaboration, communication and accountability. Clear decision making, escalation and team rules can help keep everyone on track, accountable and working collaboratively. Above all, it’s important that teams have the mindset that everyone is in it together and that they continue to look for solutions that benefit the patient and both companies. If teams do what is right for the patients, the rest is easy.
About West
West Pharmaceutical Services, Inc. is a leading manufacturer of packaging components and delivery systems for injectable drugs and healthcare products. Working by the side of its customers from concept to patient, West creates products that promote the efficiency, reliability and safety of the world’s pharmaceutical drug supply. West is headquartered in Exton, Pennsylvania, and supports its customers from locations in North and South America, Europe, Asia and Australia. West’s 2016 net sales of $1.5 billion reflect the daily use of approximately 112 million of its components and devices, which are designed to improve the delivery of healthcare to patients around the world.
