Tools & Techniques For Biopharma Root Cause Analysis
By Alex Brandon and Melissa Stappen, ValSource, Inc.

It is early Friday afternoon, and you are getting prepared to take your last work call of the week from your home office. Eager to wrap up the work week, you have already begun to daydream about your weekend plans. Without warning, you realize that your computer has lost connection to the internet with only 15 minutes to spare before the call is scheduled to begin!
Your first reflex might be to run your computer’s built-in network diagnostics tool, which informs you that your DNS server might be unavailable. Maybe your knowledge of computers does not extend this far, and so your next step might be to disable the Wi-Fi adapter on your computer and promptly reactivate it to try to reestablish the connection. This worked last time, after all. When that fix fails, restarting your computer might seem like the next appropriate step – this seems to always resolve computer gremlins! To your dismay, this method does not work either. Suddenly you recall a conversation you had with your service provider during installation of your modem and router. They mentioned that that some network connectivity issues can be resolved by disconnecting your router from its power supply, waiting 10 seconds, and then reconnecting it and allowing it to fully reboot. You decide to try this approach and, sure enough, it solves your problem, and you are back online just in time to join your work call! For many of us, this scenario is all too common and this type of troubleshooting has become second nature to us.
While the above example may seem trivial, it provides an example of how we tend to work through problem-solving in our personal lives – it can be rushed and unsystematic. Working in the biopharmaceutical industry, however, we are required to provide a complete and thorough record of our thought processes in order to allow a third party to understand how we have arrived at the root cause of a given problem.
The following mock case study will demonstrate a progression of commonly used tools and techniques that can be used to formally organize our thoughts when problem-solving in our industry. The process begins with preparation, identifying the problem, and brainstorming, and then progresses to the use of formal tools such as the fishbone diagram and five whys. The focus of this article is on the use of these tools and how they are intended to help us – not control us – throughout the problem-solving process. This article will not address the topics of correction, corrective action, or preventive action, nor the technical minutiae of the case study itself.
Case Study
On July 3, production supervisor Mike arrives on-site at XYZ Therapeutics to join his team’s shift exchange meeting prior to the start of their final shift before holiday shutdown. He enters the control room and immediately senses tension in the room among his first shift and second shift colleagues. The first shift supervisor shares the news that production has been halted due to repeated failure of a process-critical filter integrity test in the Bulk Fill Suite, whose root cause has not yet been determined. Additionally, the product’s maximum hold time at this stage is 12 hours, of which only 10 hours remain.
Preparation
Mike’s first step is to go about becoming knowledgeable about the situation. He begins by interviewing the first shift operators about the events leading up to the failure and emphasizes that his intent is not to seek blame or audit the operators but to engage in open conversation to understand the facts of the situation. Mike makes it a point to avoid leading questions, to be objective, and to not contaminate the interviews with his assumptions. Mike then asks the operators to enter the Bulk Fill Suite with him to run through a mock filter integrity test. This creates an opportunity for Mike to get eyes on the equipment and observe routine equipment operation.
Finally, Mike completes a documentation review of the historical equipment records and develops a chronology of instrument use. Mike reviews equipment records from the previous 12 months and establishes that the integrity tester had been used 13 times during this period with no recorded failures prior to its last use on June 6. Furthermore, his queries to the quality deviation tracker return no deviations associated with the instrument used. Equipped with this knowledge, Mike can now confidently lead this problem-solving effort.
Identifying The Problem
Mike’s next objective is to define the problem so that it is clear and confined to a single, observable event. Perhaps asking "what is different" between this problem situation and the "normal" non-problematic situation might be a good place to start. He focuses his investigation efforts and saves time by identifying the following descriptors:
When: July 3, final second shift prior to holiday production shutdown
Who: First and second shift production operators
What: Filter integrity test failure
Where: XYZ Therapeutics, Bulk Fill Suite
He ensures that he does not influence the problem-solving by prematurely identifying the “why” or the root cause of the problem.
Brainstorm/Fishbone Diagram
Mike assembles operators and available SMEs in the control room for a brainstorming event to begin to ascertain potential root causes for the filter integrity test failure. He decides to use the fishbone diagram (also called Ishikawa or cause and effect diagram) to identify the possible causes for the effect, or problem, and sort ideas into useful categories.1
He populates the diagram with the “What,” or the problem identified previously, and establishes the categories applicable to this problem-solving event. Method, Material, Measurement, Machine, People/Personnel, and Environment are commonly used categories; however, these can and should be tailored to the circumstances surrounding individual problems. See Diagram 1 for an example template.2
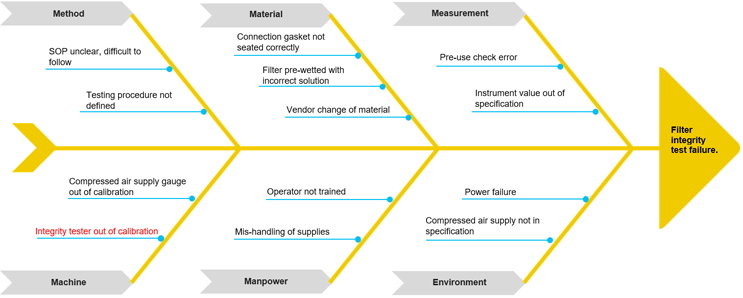
Diagram 1: Fishbone Diagram
At this point, Mike needs to evaluate which causes to further investigate. For this, he must review each potential cause and eliminate from his investigation those that have data and evidence in place to support not further investigating them. In this way, he narrows the investigation down to the most probable root cause(s). For example, the cause “operator not trained” can be eliminated from the investigation by reviewing training records and confirming operator qualification on the associated procedures.
Five Whys
For those causes lacking data and evidence that can exclude them from the investigation, the five whys tool is used to arrive at a true root cause.
Five whys is a technique for discovering the root causes of a problem and showing the relationship of causes by repeatedly asking the question, “Why?” It’s a repetitive questioning technique to probe deeper to surface the root cause of a problem. The number of times “why” is asked depends on when the true root cause is reached through an iterative line of questioning that underlines cause and effect relationships.3 As with any tool, five whys has its nuances. For instance, once you begin down a specific line of questioning, it can become difficult to redirect and look at alternative paths. For this reason, it is important to maintain flexibility throughout the use of this tool.
After using data and evidence to eliminate several causes from his investigation, Mike is left with one probable cause to evaluate using the five whys tool. See Diagram 2 below.
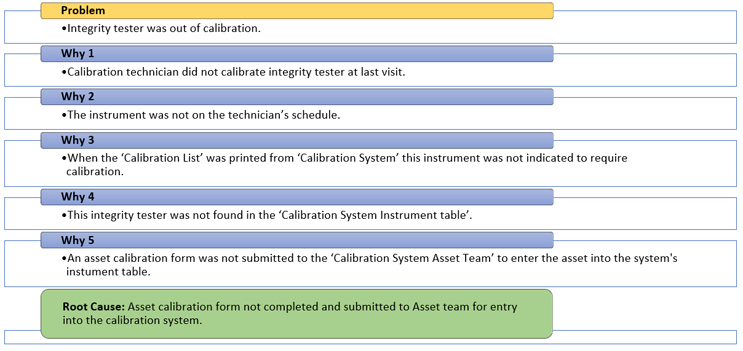
Diagram 2: 5 Whys – “Machine”
Actions
Mike has successfully identified an actionable true root cause for the filter integrity test failure.
Root causes are the specific underlying causes of a problem, can be reasonably identified, are under the control of management to fix, and allow for generation of effective recommendations to correct and prevent them.4
Mike’s next step is to determine appropriate and effective actions to take to address these root causes. Actions can include correction, corrective action (CA), and preventive action (PA). See definitions below.
- Correction: a remedy to fix what was affected by the incident if possible4
- Corrective Action: action to eliminate the cause of a detected nonconformity or other undesirable situation4
- Preventive Action: action to eliminate the cause of a potential nonconformity or other undesirable situation4
Closing Thoughts
As highlighted in the opening two paragraphs of this article, when presented with problems in our personal lives, intellectual inertia often drives us to adopt quick fixes rather than spending the time required to thoroughly investigate problems. This can lead to false diagnoses and ultimately ineffective or incomplete solutions to these problems. As we are presented with unexpected conditions and unplanned events in our work environment, the tools and techniques presented above can be used to organize our thoughts, conduct unbiased investigations, arrive at true root cause, and document problem-solving activities.
While the case study above presented a linear progression through specific techniques and tools, real-life investigations are not always this formulaic and unambiguous. For example, Mike may have considered using the five whys tool during brainstorming, and then gone on to find evidence. Alternatively, he could have investigated a “why,” collected evidence (or found none), and then moved on to the next “why” or moved to a completely different category of “why.” It is important to underscore the need for flexibility when conducting this type of problem-solving activity. Ultimately, the tools and techniques discussed above can be used together or separately, but the outputs of the process should allow for information to be documented in a manner that is compliant with applicable procedures and regulations.
References
- Tague, Nancy R. (2004). "Seven Basic Quality Tools". The Quality Toolbox. Milwaukee, Wisconsin: American Society for Quality.
- “25 Great Fishbone Diagram Templates & Examples [Word, Excel, Ppt].” TemplateLab, 14 June 2022, https://templatelab.com/fishbone-diagram-templates/.
- “Letter F - Quality Glossary of Terms, Acronyms & Definitions with Letter F.” ASQ, https://asq.org/quality-resources/quality-glossary/f.
- Root Cause Investigations for CAPA: Clear and Simple by James Vesper (June 8, 2020)
 About The Authors:
About The Authors:
Alex Brandon is a MSAT analyst at ValSource, Inc. with five years of biopharmaceutical experience across biologics and cell therapies. His background includes commercial cGMP protein manufacturing operations; manufacturing facility start-up activities; and commissioning, qualification, and validation of manufacturing systems.
 Melissa Stappen is a senior consultant with ValSource, Inc., with over 30 years of experience in the pharmaceutical, medical device, and biotech industry and clinical/healthcare provider settings. During her career, she has provided support to quality assurance, quality control, and compliance departments as a subject matter expert relating to endotoxin and microbial contamination control. You can email her at mstappen@valsource.com.
Melissa Stappen is a senior consultant with ValSource, Inc., with over 30 years of experience in the pharmaceutical, medical device, and biotech industry and clinical/healthcare provider settings. During her career, she has provided support to quality assurance, quality control, and compliance departments as a subject matter expert relating to endotoxin and microbial contamination control. You can email her at mstappen@valsource.com.
