Understanding The Microfluidic Platforms For Scaling Nanoparticle Production
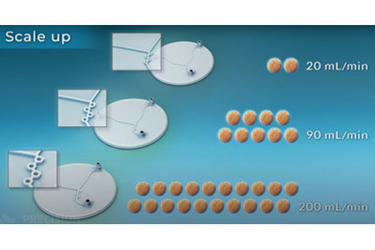
COVID-19 vaccines have made RNA lipid nanoparticles (LNPs) known worldwide. The production of RNA-LNP with microfluidic involves mixing RNA in an aqueous buffer with lipids dissolved in ethanol in a non-turbulent, exquisitely controlled manner, resulting in consistent and reproducible LNPs. The process maximizes drug loading, particle quality, and the scope of compositions possible with fully scalable results. Microfluidics can generate LNPs with precisely defined properties but have been limited by challenges in scaling throughput. For the development of RNA therapeutics, the challenge is the development of formulations that require sophisticated and innovative drug delivery nanoparticles consistent across the various scales of drug development.
Learn about the benefits of microfluidics for nanoparticle production over traditional methods as well as scaling nanoparticle production for clinical or industrial use.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Biosimilar Development? Subscribe today.
