When The Goal Is To Be (Bio)similar, What Does Differentiation Mean?
By Mark Langston, Boehringer Ingelheim Pharmaceuticals, Inc.

As concerns about the cost of medicines reach a fever pitch, innovators are looking to leverage a promising advancement to help address the fundamental need for sustainable healthcare in the U.S. — biosimilars.
Biosimilars are highly similar versions of already approved biologic medicines with no clinically meaningful differences in terms of safety, potency, and purity. For more than a decade in Europe, biosimilars have helped increase access to important biologic medicines at a lower cost. There are now nearly 30 biosimilars available in Europe.1 Despite their success abroad and pricing reductions of 20 to 30 percent less than their reference biologics,2 biosimilar adoption here in the U.S. remains uncertain.
As of September 2017, seven biosimilars have been approved by the FDA,3 only three of which are currently available t0 patients. Ongoing litigation remains a top reason for the delay of market entry for biosimilars, but significant knowledge gaps around these medicines and their potential cost benefit to the healthcare system will present a critical barrier to acceptance once they are made available.
The Biosimilars Forum, a trade organization that informs and supports public policies to encourage awareness, access, and adoption of biosimilars, found that the majority of specialty physicians have heard about biosimilars, but they still have limited understanding of how biosimilars work and how their equivalency to an existing biologic is determined (see Figure 1).
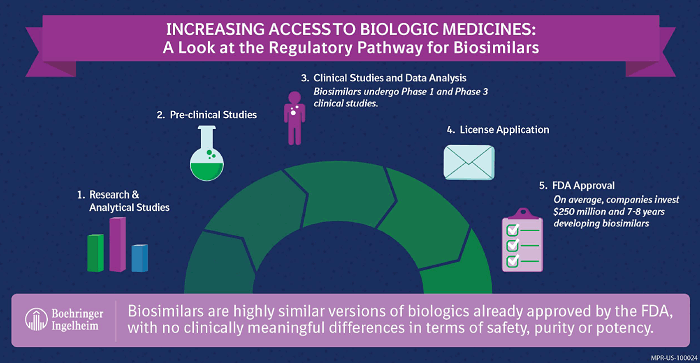
Figure 1
In order for biosimilars to reach their full potential in the U.S., they first need to be better understood and accepted by healthcare professionals and payors. Facilitating this understanding will present a unique challenge for the pharmaceutical industry, among other stakeholders.
When it comes to marketing, a pharmaceutical company’s efforts are typically focused on differentiating its products from competitor treatments already available on the market. For most new medicines, a company aims to help patients and the healthcare community understand the therapeutic and outcomes benefits versus an existing standard, but for biosimilars the challenge is how to help stakeholders understand the concept of biosimilarity. In addition, companies have to build confidence that biosimilars truly have no clinically meaningful differences in safety, potency, and purity from their reference biologic.
As biosimilars advance on the U.S. market, companies are grappling with several key questions including:
- Will biosimilars be marketed similarly to branded medicines, or will other elements like manufacturing expertise, clinical data package for multiple reference product indications, and the rigorous testing conducted to establish biosimilarity need to be presented?
- How important is patient education in biosimilar marketing plans, particularly in an age where patients are more empowered to self-research and drive conversations with physicians around their healthcare?
- What is the best approach to ensuring payors and prescribers understand the robust analysis required to demonstrate biosimilarity, when they are accustomed to making decisions based on the current clinical development and regulatory pathway for a branded biologic?
- How much emphasis should be placed on commercial differentiation versus biosimilarity to a reference product, not to mention differentiation among biosimilars for the same reference biologic?
- Will differentiation among biosimilars depend on the breadth of data that each sponsor generates for a given biosimilar candidate? How much and what data will impact which biosimilar brands rise to the top?
- How important will a biosimilar developer’s approach to the overall patient experience be in differentiating their products from other biosimilars?
- How important is the extent of biologic manufacturing experience of the biosimilar manufacturer?
- According to a recent survey, nearly 80% of physician respondents said they would be more comfortable switching a stable patient from a branded product to a biosimilar product if the biosimilar in question was designated by the FDA as an “interchangeable” biosimilar.4 While the approval of interchangeable biosimilars should not imply approved non-interchangeable biosimilars as inferior, could interchangeability status distinguish some biosimilars from others?
At Boehringer Ingelheim, we hope that finding successful approaches to these issues will lead to confidence among payors and healthcare professionals that is needed to expand access to innovative medicines like biosimilars while saving billions5 of dollars for patients and the healthcare system.
References:
- Biosimilars in Europe. (2017, May 10). Retrieved from http://www.biopharma-reporter.com/Markets-Regulations/Biosimilars-in-Europe-11-years-28-approvals-0-safety-concerns.
- Greater Potential Cost Savings with Biosimilar Use. (2016, May 17). Retrieved from http://www.ajmc.com/journals/issue/2016/2016-vol22-n5/greater-potential-cost-savings-with-biosimilar-use.
- FDA Clears Biotech Drug Copycats, But Buying Them Isn’t So Easy. (2017, September 18). Retrieved from https://www.bloomberg.com/news/articles/2017-09-18/fda-clears-biotech-drug-copycats-but-buying-them-isn-t-so-easy.
- Physician Views Poll Results – The Potential Impact of U.S. Biosimilar Interchangeability in Five Charts. (2017, August 8). Retrieved https://www.firstwordpharma.com/footer/benefits?tsid=17.
- American Journal of Pharmacy Benefits –Biosimilars Could Save Billions Over the Next Decade. (2017, July 14). Retrieved http://www.ajpb.com/news/biosimilars-could-save-billions-over-the-next-decade
About The Author:
 Mark Langston is executive director of biosimilar marketing at Boehringer Ingelheim Pharmaceuticals, Inc. One of his areas of specialization is commercial strategy development; in addition, he has worked in regional and country management roles around the world. Mark has successfully launched specialty medicines for Boehringer Ingelheim and is currently leading the company’s commercialization of its biosimilar portfolio. He began his career in healthcare as a cardiothoracic intensive care nurse in the National Health Service in the UK.
Mark Langston is executive director of biosimilar marketing at Boehringer Ingelheim Pharmaceuticals, Inc. One of his areas of specialization is commercial strategy development; in addition, he has worked in regional and country management roles around the world. Mark has successfully launched specialty medicines for Boehringer Ingelheim and is currently leading the company’s commercialization of its biosimilar portfolio. He began his career in healthcare as a cardiothoracic intensive care nurse in the National Health Service in the UK.
