Where Do We Stand On Adopting Continuous Manufacturing For Biologics?
By Richard Chen, Chris Hwang, Kelvin Lee, Andrew Zydney, and John F. Kokai-Kun

Continuous manufacturing (CM) is a way of producing goods in which raw materials are constantly fed into the production process and move through it without any meaningful discernable discrete transitions between the transformation of inputs into outputs. 1 CM offers several advantages over the alternative approach, batch manufacturing, such as steady-state operation, reduced equipment size, high-volumetric productivity, short cycle times, and reduced capital costs. 2 This has led many companies across several industries, including food processing, steel manufacturing, and chemical and petrochemical production, to transition their production processes to CM. 3 However, due to a combination of technical and regulatory challenges, the pharmaceutical industry has not been as quick to adopt CM en masse. 3,4
The list of pharmaceuticals manufactured using CM is short (Table 1 below). As of 2022, only 13 drugs made using CM are approved in Europe, Japan, and the United States, and several of these drugs, such as Pfizer's oncology drug Daurismo (glasdegib), were initially approved with a batch production process and then transitioned to CM afterward. 5-7
Table 1. FDA approved drugs made using continuous manufacturing, 2015 to 2021
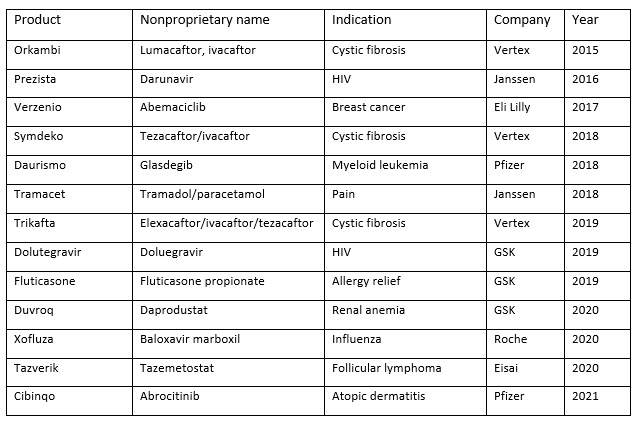
The lack of approved products made with CM does not reflect the significant effort currently underway to adapt this approach to manufacturing drugs. Many companies are looking to add CM processes to their product pipelines and collaborating with academic institutions to innovate continuous processes. 8 As these efforts bear fruit, the industry is also starting to look toward how to adapt CM to manufacturing biologics, which account for most of the new therapies that enter the market each year. 9,10 It is worth noting that there is debate as to whether all biologic products are amenable to CM.11
Many biologic manufacturing processes start with cell culture to produce the biologic product, for which a couple of different approaches exist. The most common method is fed-batch, which uses large tanks to culture cells for up to three weeks before the cells eventually consume all the nutrients and overwhelm the media with waste products. However, fed-batch bioreactors were not always the preferred approach. In the early days of biopharmaceutical manufacturing, lower cell line productivity favored the use of perfusion bioreactors that can maintain cells for months by continuously feeding them with fresh nutrients while at the same time removing spent media and product. 12 Perfusion bioreactors are also ideal for manufacturing labile proteins, such as recombinant enzymes or coagulation factors, where continuous harvest is better for optimal product quality. 13,14 Fed-batch bioreactors are easier to operate and scale up, however, so they replaced most perfusion manufacturing as their productivity improved from less than 1 gram per liter to more than 4 grams per liter for some products.
CM Gives New Purpose To Older Technology
Perfusion is not entirely gone, and some biologics are currently made using this process (Table 2). Perfusion has also been experiencing a resurgence of interest within the industry with an eye toward developing continuous processes. 15-18 This is not surprising since process output from perfusion cell culture has been demonstrated to be significantly higher than that of conventional fed-batch processes due to significantly higher cell densities and a decrease in bioreactor downtime. Furthermore, perfusion processes require a much smaller facility footprint than fed-batch. 19 These benefits can be significant for manufacturing any biologic but are especially relevant for products targeting relatively small patient populations for which large up front capital expenditures on equipment and space may not make economic sense. For example, Sanofi has demonstrated the commercial feasibility of using integrated continuous perfusion for their rare disease enzyme replacement pipeline, which includes Nexviazyme (avalglucosidase alfa-ngpt) for late-onset Pompe disease. 20
Table 2. mAbs produced by continuous manufacturing
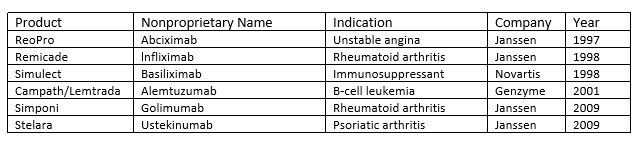
Select sample of licensed mAb products manufactured using continuous perfusion approved by the FDA between 1993 and 2014. 21
Using a perfusion bioreactor can also improve product quality by maintaining a uniform microenvironment during production while minimizing the variability of residence times for the product. For example, monoclonal antibodies (mAbs) secreted by Chinese hamster ovary (CHO) cells at the start of a batch cell culture are released into a nutrient-rich environment that includes few lysed cells. In a batch process, where an antibody remains in the bioreactor for multiple days before harvest, the conditions can change dramatically between the start and finish as media is consumed and waste products and dead cells accumulate. Several studies have shown a lot of the glycosylation, deamidation, and aggregation variability of mAbs is caused by the wide range of residence times inherent to batch processes. 22 In a continuous perfusion process, the mAbs are removed from the culture and progress into downstream processing soon after forming, reducing extended exposure to the changing cell culture conditions found in a batch or fed-batch reactor. Residence time in the production reactor is even more of an issue for less stable products with shorter half-lives like some cellular therapies.
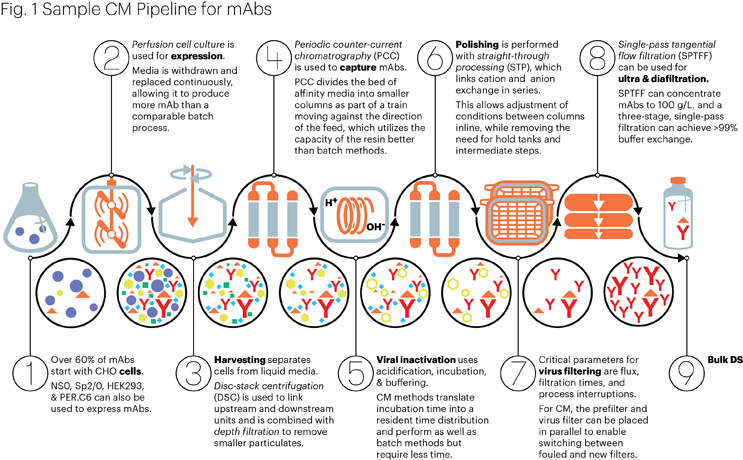
(Figure by Mark Verdecia of USP.)
Implementing a perfusion process is not simply a matter of extending the run length, however. Doing so offers no real advantage if the perfusion process is not robust and highly productive because the yield for a given space and time will still be low. Optimizing perfusion processes is, therefore, necessary to achieve maximum yields. Recently, Transcenta achieved a volumetric productivity of greater than 6 g/L per day using a proprietary continuous perfusion cell culture platform for a four-week culture. 23 This improvement is a 15-fold increase in output, allowing one 500 L single-use bioreactor to produce approximately 600 kg of total drug substance each year. Others, including Sartorius Stedim Biotech, have reported using a 100 L perfusion bioreactor to achieve the yields expected from a 5,000 L fed-batch bioreactor. 24
Additional Innovations Showing Promise To Aid Adoption
Other innovations, including separation technologies such as tangential flow filtration (TFF) and alternating tangential flow (ATF) filtration, are also impacting the industry. The lower shear stress of ATF allows manufacturers to extract the spent media containing the protein of interest while returning a much higher percentage of cells safely back into the bioreactor. 25 This helps the process maintain higher cell densities and reduces the need for additional clarification steps. 26
The future of CM for biologics is not just about cell culture. Integrating upstream and downstream unit operations into either complete end-to-end CM or partial CM can achieve increases in efficiency that would translate into significant reductions in costs. An integrated continuous biomanufacturing platform has been reported to reduce processing costs by 55% relative to conventional batch operations for mAb production. 27 More substantial reductions in operational costs and a three-fold decrease in capital expenditures are possible for non-mAb products. 21 A few companies have reportedly made this leap. BiosanaPharma has reported a fully continuous manufacturing process for a mAb currently in Phase 1 clinical trials, and Enzene Biosciences recently opened a fully continuous biologics manufacturing facility in India. 28,29
Many barriers to adopting CM for biologics are inherent to the technology needed for product purification. For example, bind-and-elute chromatography is more conducive to batch manufacturing than to CM, and final formulation conditions are often only achieved at the end of a batch diafiltration process which may not necessarily be conducive to CM. Significant progress has been made to address these technological limitations, however. Solutions include flow chromatography systems and countercurrent staged diafiltration processes, as well as automated column and filter switching systems. 30,31
Other developments that can help with the adoption of integrated CM are appropriate process analytical technologies (PAT) for rapid in-line or at-line evaluation of critical process parameters and critical quality attributes to ensure that the process remains in control. Using PAT can be very beneficial to speed up process development, improve process understanding, and establish a robust control strategy. However, PAT is still immature, especially for manufacturing larger molecules such as mAbs. While in-line measurements of pH, UV, and conductivity are easily implemented, the rapid analysis of other parameters, such as aggregation, bioburden, and glycosylation are more challenging and may require developing new technologies and methods. Fortunately, while the industry waits for these technologies to come online, it can still realize many of the benefits of CM, including increased productivity, shorter process development times, reduced operational costs, minimal human error, and a smaller manufacturing footprint.
Despite these efforts, the number of known biologics manufacturers implementing CM processes that link or integrate operations is still small, even if it has grown significantly over the last five years.
Manufacturers Need To Consider Whether CM Is Right For Them
Before adopting CM, manufacturers must evaluate the costs, business case, and technical feasibility of the available equipment and facility for producing the products in their pipeline.
If you choose to adopt CM, you will need to shift your mindset with new ways of thinking about biologics production. Product quality variability still needs to be evaluated across multiple lots or across multiple days of culture of the bioreaction process. In batch manufacturing, the lots are defined by the amount of material produced, but CM can define batches in various ways, including by time rather than the amount of bulk drug produced when all unit operations are complete. Therefore, manufacturers that want to implement CM must make procedural changes within their quality systems to enable flexibility in defining batches. Manufacturers also need to understand how transient events can impact an individual unit operation and the entire CM process. Understanding the impact of transient events is critical to identifying product quality risks, whether these are planned events (such as process start-ups, pauses, or shutdowns) or unplanned events. Dealing with these issues requires a robust control strategy because CM requires rapid real-time decision-making to keep the process in control and automation that can respond quickly and with some flexibility to changing conditions. A crucial element of a CM control strategy is diverting out-of-specification (OOS) material in real time to help maintain overall product quality.
As already mentioned, several economic and operational reasons exist for adopting CM, but manufacturers in the pharmaceutical industry must also comply with very high levels of regulatory oversight. Getting regulatory approval for a biologic product is already time-consuming and resource-intensive. Manufacturers understandably have concerns about anything that could delay the approval and commercialization of their products, even though regulators have acknowledged that CM can improve quality, enhance efficiency, address shortages of medical products, and speed time-to-market. 32
Knowledge Sharing And Regulator Input Will Spur CM Adoption
The lack of scientific and regulatory guidance for the development, implementation, operation, and life cycle management of continuous processes for biologics creates risk. Current guidance, including ICH Q8, Q9, Q10, and Q11, may be well understood for batch manufacturing processes, but their implementation for CM is still evolving. 33-36 More specific guidance is forthcoming, including ICH Q13, which focuses on CM for therapeutic proteins. 37 However, the industry requires more input from regulators, and regulators need more information from the industry. Fortunately, the FDA is prioritizing advanced manufacturing and adding staff to its Emerging Technology Program, which assesses continuous, modular, and smart manufacturing.
Industry organizations are also starting to take an active role in fostering the use of CM. The National Institute for Innovation in Manufacturing Biopharmaceuticals facilitated a case study on mAb manufacturing that aims to provide a shared understanding on the implementation of control strategies in CM for mAbs. 38,39 The industry organization, BioPhorum, also has a workstream on Continuous Bioprocessing as part of its Biomanufacturing Technology Roadmap.40
The industry needs facilitators to help spur the creation and sharing of knowledge that will allow regulatory authorities to properly balance risk and operational efficiency while enabling manufacturers to make the right decisions when implementing CM. Precompetitive collaboration and knowledge sharing between all parties involved will help the industry obtain a critical mass of knowledge more quickly, which is needed to secure the technology's current benefits.
Acknowledgments:
We would like to thank Dr. Irina Ramos for reviewing the manuscript and providing feedback with manuscript revisions and Dr. Mark Verdecia for assistance with preparing the manuscript.
References
- Fisher, A. C. et al. An audit of pharmaceutical continuous manufacturing regulatory submissions and outcomes in the US. Int J Pharm 622, 121778 (2022). https://doi.org:10.1016/j.ijpharm.2022.121778
- Konstantinov, K. B. & Cooney, C. L. White Paper on Continuous Bioprocessing May 20–21 2014 Continuous Manufacturing Symposium. Journal of Pharmaceutical Sciences 104, 813-820 (2015). https://doi.org:10.1002/jps.24268
- Lee, S. L. et al. Modernizing Pharmaceutical Manufacturing: from Batch to Continuous Production. Journal of Pharmaceutical Innovation 10, 191-199 (2015). https://doi.org:10.1007/s12247-015-9215-8
- Rossi, C. V. A Comparative Investment Analysis of Batch Versus Continuous Pharmaceutical Manufacturing Technologies. J Pharm Innov, 1-19 (2022). https://doi.org:10.1007/s12247-021-09612-y
- Badman, C. et al. Why We Need Continuous Pharmaceutical Manufacturing and How to Make It Happen. Journal of Pharmaceutical Sciences 108 (2019). https://doi.org:10.1016/j.xphs.2019.07.016
- Kansteiner, F. End-to-end: Can pharma finally make the dream of continuous manufacturing a reality?, (2021).
- Pfizer. Deliver First-In-Class Science, (2019).
- Trafton, A. Continuous drug manufacturing offers speed, lower costs, (2012).
- Klutz, S. et al. Developing the biofacility of the future based on continuous processing and single-use technology. J Biotechnol 213, 120-130 (2015). https://doi.org:10.1016/j.jbiotec.2015.06.388
- Farid, S. S., Thompson, B. & Davidson, A. Continuous bioprocessing: the real thing this time? 10(th) Annual bioProcessUK Conference, December 3-4, 2013, London, UK. MAbs 6, 1357-1361 (2014). https://doi.org:10.4161/mabs.36151
- Walther, J. et al. The business impact of an integrated continuous biomanufacturing platform for recombinant protein production. J Biotechnol 213, 3-12 (2015). https://doi.org:10.1016/j.jbiotec.2015.05.010
- Lehr, B. & Lyons, D. Perfusion in the 21st Century, (2016).
- Desai, S. G. Continuous and semi-continuous cell culture for production of blood clotting factors. J Biotechnol 213, 20-27 (2015). https://doi.org:10.1016/j.jbiotec.2015.02.021
- Gjoka, X., Gantier, R. & Schofield, M. Transfer of a three step mAb chromatography process from batch to continuous: Optimizing productivity to minimize consumable requirements. J Biotechnol 242, 11-18 (2017). https://doi.org:10.1016/j.jbiotec.2016.12.005
- Pollock, J., Coffman, J., Ho, S. V. & Farid, S. S. Integrated continuous bioprocessing: Economic, operational, and environmental feasibility for clinical and commercial antibody manufacture. Biotechnol Prog 33, 854-866 (2017). https://doi.org:10.1002/btpr.2492
- Rodrigues, M. E., Costa, A. R., Henriques, M., Azeredo, J. & Oliveira, R. Technological progresses in monoclonal antibody production systems. Biotechnol Prog 26, 332-351 (2010). https://doi.org:10.1002/btpr.348
- Godawat, R., Konstantinov, K., Rohani, M. & Warikoo, V. End-to-end integrated fully continuous production of recombinant monoclonal antibodies. J Biotechnol 213, 13-19 (2015). https://doi.org:10.1016/j.jbiotec.2015.06.393
- Zydney, A. L. Continuous downstream processing for high value biological products: A Review. Biotechnol Bioeng 113, 465-475 (2016). https://doi.org:10.1002/bit.25695
- Walker, N. Perfusion Appears to be Gaining Traction in Biopharma Manufacturing, (2017).
- Lu, J. et al. in Cell Culture Engineering XVI. (eds A. Robinson, R. Venkat, & E. Schaefer).
- Erickson, J. et al. End-to-end collaboration to transform biopharmaceutical development and manufacturing. Biotechnol Bioeng (2021).
- Le, H., Chen, C. & Goudar, C. T. Continuous Processing in Upstream Operations. Chemical Engineering Progress 111, 32-40 (2015).
- Transcenta Continues to Push the Boundary of Cell Culture Productivity by Achieving > 6 g/L PER Day Volumetric Productivity in Continuous Perfusion Platform (2021).
- Zijlstra, G. & Gupta, P. Supplement: Moving toward Continuous Bioprocessing. Genetic Engineering & Biotechnology News 37 (2017).
- Radoniqi, F., Zhang, H., Bardliving, C. L., Shamlou, P. & Coffman, J. Computational fluid dynamic modeling of alternating tangential flow filtration for perfusion cell culture. Biotechnol Bioeng 115, 2751-2759 (2018). https://doi.org:10.1002/bit.26813
- Kelly, W. et al. Understanding and modeling alternating tangential flow filtration for perfusion cell culture. Biotechnol Prog 30, 1291-1300 (2014). https://doi.org:10.1002/btpr.1953
- Walther, J. et al. The business impact of an integrated continuous biomanufacturing platform for recombinant protein production. J Biotechnol 213, 3-12 (2015). https://doi.org:10.1016/j.jbiotec.2015.05.010
- Editorial. First mAb Produced via Fully Continuous Biomanufacturing. Genetic Engineering & Biotechnology News (2019).
- Enzene. Enzene Opens its First Continuous Biologics Manufacturing Facility With a Promise to Disrupt the mAb Manufacturing Cost, (2019).
- Dutta, A. K., Tan, J., Napadensky, B., Zydney, A. L. & Shinkazh, O. Performance optimization of continuous countercurrent tangential chromatography for antibody capture. Biotechnol Prog 32, 430-439 (2016). https://doi.org:10.1002/btpr.2250
- Jabra, M. G., Yehl, C. J. & Zydney, A. L. Multistage continuous countercurrent diafiltration for formulation of monoclonal antibodies. Biotechnol Prog 35, e2810 (2019). https://doi.org:10.1002/btpr.2810
- FDA. Advancing Regulatory Science at FDA. (U.S. Food and Drug Administration, 2021).
- ICH. ICH guideline Q8 (R2) on pharmaceutical development. (International Committee on Harmonization, 2017).
- ICH. Q9 Quality Risk Management. (International Conference on Harmonisation, 2005).
- ICH. Q10: Pharmaceutical Quality System. (International Conference on Harmonisation).
- ICH. Q11: Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities). (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2012).
- ICH. Q13: Continuous Manufacturing of Drug Substances and Drug Products. (International Council for Harmonisation, 2021).
- NIIMBL. Project Call 5.2, (2022).
- NIIMBL. N-mAb: A case study to support development and adoption of integrated continuous bioprocesses for monoclonal antibodies. (National Institute for Innovation in Manufacturing Biopharmaceuticals, 2022).
- BioPhorum. Technology Roadmap Vision 2.0. Continuous downstream processing for biomanufacturing: an industry review. (2022)
About The Authors:
Richard Chen is senior research advisor, purification & virology, Lilly Research Laboratories, at Eli Lilly and Company.
Chris Hwang is EVP and chief technology officer at Transcenta Holding Ltd., USA
Kelvin Lee is institute director, National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL). He is also Gore Professor, Chemical & Biomolecular Engineering Dept., Delaware Biotechnology Institute, University of Delaware.
Andrew Zydney is Bayard D. Kunkle Chair and professor of chemical engineering, Department of Chemical Engineering, The Pennsylvania State University.
John F. Kokai-Kun is director of external scientific collaboration in the Department of Global Biologics, The United States Pharmacopeia.
